Wednesday, January 31, 2007
Wednesday, Day 7
Honors: took our bonding/IMFA test; results and analysis by early next week; some of you are going to have to correct your corrections from the last test; I will not be giving that opportunity again: get it right the first time; if not, get it right the second time but that is the limit.
Regents: Today, we began, in earnest, our NEW unit: the Math of Chemistry. We learned how to balance an equation by mass and charge; we will be doing many more examples of this problem type on Friday. We then looked at three different types of chemical reaction: synthesis, decomposition, and single replacement. On Friday, we will learn two more common reaction types. Tomorrow is your third and final bonding/intermolecular forces exam. Serious practice and study is required for this unit especially after the lab exam debacle.
I will be in at around 7:30AM if you have any last minute questions tomorrow.
AP: we wrote equilibrium constant EXPRESSIONS in terms of concentrations, Kc, and also in terms of partial pressures, Kp.
We also practiced with the formula that interrelates Kc and Kp; that formula is in your reference table. What is NOT in the table is how to calculate "delta n" in that formula; we discussed that in class.
We then looked at a heterogeneous solid-gas equilibrium and saw the disturbing and surprising result that AMOUNTS of SOLIDS are IRRELEVANT to EQUILIBRIUM GAS concentrations or pressures because SOLIDS are NOT in the equilibrium constant EXPRESSION! More on this empirically discovered fact tomorrow.
For tonight, start on Chapter 15 with the first section.
Regents: Today, we began, in earnest, our NEW unit: the Math of Chemistry. We learned how to balance an equation by mass and charge; we will be doing many more examples of this problem type on Friday. We then looked at three different types of chemical reaction: synthesis, decomposition, and single replacement. On Friday, we will learn two more common reaction types. Tomorrow is your third and final bonding/intermolecular forces exam. Serious practice and study is required for this unit especially after the lab exam debacle.
I will be in at around 7:30AM if you have any last minute questions tomorrow.
AP: we wrote equilibrium constant EXPRESSIONS in terms of concentrations, Kc, and also in terms of partial pressures, Kp.
We also practiced with the formula that interrelates Kc and Kp; that formula is in your reference table. What is NOT in the table is how to calculate "delta n" in that formula; we discussed that in class.
We then looked at a heterogeneous solid-gas equilibrium and saw the disturbing and surprising result that AMOUNTS of SOLIDS are IRRELEVANT to EQUILIBRIUM GAS concentrations or pressures because SOLIDS are NOT in the equilibrium constant EXPRESSION! More on this empirically discovered fact tomorrow.
For tonight, start on Chapter 15 with the first section.
Tuesday, January 30, 2007
Tuesday, Day 6
AP: Kinetics test with a record "k" parts to a question. Nice. Can't wait to discuss the power of Excel and we still can apply regression analysis to the van'tHoff equation in the equilibrium unit. That equation is practically identical to the Claus-Clap and the Arrhenius equations.
Regents: we finished our intermolecular attraction/bonding unit by discussing the various types of solids and their respective physical properties. Our test on Thursday will focus on material from the past two weeks.
Tomorrow, we begin our new unit: the Math of Chemistry!
Honors: we reviewed balancing and reaction types; then we went on to the most important lesson of the second half of the course: the Magic Triangle, with which we will be able to inter-convert moles, grams, particles, and even liters of gas for any substance! A little more to be revealed and much to be practiced after our test tomorrow until you are all magic triangle WIZARDS.
Good luck tomorrow. See post below on what NOT to do.
Regents: we finished our intermolecular attraction/bonding unit by discussing the various types of solids and their respective physical properties. Our test on Thursday will focus on material from the past two weeks.
Tomorrow, we begin our new unit: the Math of Chemistry!
Honors: we reviewed balancing and reaction types; then we went on to the most important lesson of the second half of the course: the Magic Triangle, with which we will be able to inter-convert moles, grams, particles, and even liters of gas for any substance! A little more to be revealed and much to be practiced after our test tomorrow until you are all magic triangle WIZARDS.
Good luck tomorrow. See post below on what NOT to do.
Mistakes to avoid
I do NOT want to rant after tomorrow's test. So, HEED THIS (ANTI-)ADVICE!
Instead of repeating the same explanation over again, here is a list of things NOT to write on the Honor's exam. The following are a list of absolutely unbelievable distortions that I NOT ONLY have explicitly showed you how to avoid and WHY you should avoid them (because they are patently ridiculous) BUT ALSO that I have never ever even remotely suggested them as legitimate phenomena or explanations:
1. NEVER EVER EVER call an F-H, O-H, or N-H covalent bond WITHIN a given molecule a "hydrogen bond". ANY BOND that involves the SHARING of electrons BETWEEN TWO nonmetal nuclei is BY DEFINITION a COVALENT BOND!
(Hydrogen "bonding" is merely an intermoleculare attraction, NOT BETWEEN ATOMS, but between SEPARATE and DISTINCT molecules; each molecule MUST have an F-H, O-H, or N-H covalent bond WITHIN the molecule in order to be polar enough to have extreme dipole-dipole attractions.
2. NEVER call hydrogen bonding attractions MERELY dipole-dipole attractions. THERE NEVER WOULD EVEN BE a separate category CALLED hydrogen bonding attractions if they were not (INTENSELY) EXTREME dipole-dipole attractions.
3. NEVER EVER EVER WRITE THAT IONIC BONDING HAS ANYTHING TO DO WITH ZEFF OR ELECTRONS!!! IONIC bonds ARE BONDS between and AMONG OPPOSITELY CHARGED IONS (CATIONS AND ANIONS), NOT ELECTRONS SHARED BETWEEN NUCLEI!!!
YOU WILL SEE ionic bonds form EVERY TIME there is a compound of a METAL AND A NONMETAL and NEVER in a compound of NON-METAL and a NONMETAL.
4. NEVER say that a nonpolar molecule is NOT ATTRACTED TO or (even worse!) REPELLED by a polar molecule! ALL MOLECULES ATTRACT EACH OTHER BUT polar molecules attract other polar molecules to a greater degree BECAUSE polar molecules have PERMANENT and relatively GREATER partial negative and partial positive regions to each molecule than do nonpolar molecules.
5. IF YOU BOTHER to take the time to draw a picture of what you are talking about, REFER TO YOUR DRAWING REPEATEDLY THROUGHOUT YOUR EXPLANATION!!! EVERY SINGLE TEST, TIME AFTER TIME, PEOPLE FORGET TO DO THIS AND, AS A RESULT, THEY LOSE CRAZY POINTS EVERY TIME!!!
6. DO NOT WRITE OUT YOUR ENTIRELY REHEARSED ANSWERS IF THEY ARE NOT RELATED TO THE QUESTION!!! NO WONDER YOU RUN OUT OF TIME!!! I cannot tell you how many students wrote paragraphs of irrelevant facts such as explaining that "solid salts do not conduct electricity" EVEN THOUGH SUCH A QUESTION DID NOT REMOTELY EXIST ON THE LAST TEST!
7. IF you do not yet know how to go from formula to name and vice-versa for any type of compound that we have covered so far, then you are going to lose A LOT of points on tomorrow's test. You cannot do math of chem or any kind of chemical equations unless you know how to name compounds and how to write formulas from names.
MAKE ABSOLUTELY SURE that you are bulletproof on naming or you are not going to do well.
Instead of repeating the same explanation over again, here is a list of things NOT to write on the Honor's exam. The following are a list of absolutely unbelievable distortions that I NOT ONLY have explicitly showed you how to avoid and WHY you should avoid them (because they are patently ridiculous) BUT ALSO that I have never ever even remotely suggested them as legitimate phenomena or explanations:
1. NEVER EVER EVER call an F-H, O-H, or N-H covalent bond WITHIN a given molecule a "hydrogen bond". ANY BOND that involves the SHARING of electrons BETWEEN TWO nonmetal nuclei is BY DEFINITION a COVALENT BOND!
(Hydrogen "bonding" is merely an intermoleculare attraction, NOT BETWEEN ATOMS, but between SEPARATE and DISTINCT molecules; each molecule MUST have an F-H, O-H, or N-H covalent bond WITHIN the molecule in order to be polar enough to have extreme dipole-dipole attractions.
2. NEVER call hydrogen bonding attractions MERELY dipole-dipole attractions. THERE NEVER WOULD EVEN BE a separate category CALLED hydrogen bonding attractions if they were not (INTENSELY) EXTREME dipole-dipole attractions.
3. NEVER EVER EVER WRITE THAT IONIC BONDING HAS ANYTHING TO DO WITH ZEFF OR ELECTRONS!!! IONIC bonds ARE BONDS between and AMONG OPPOSITELY CHARGED IONS (CATIONS AND ANIONS), NOT ELECTRONS SHARED BETWEEN NUCLEI!!!
YOU WILL SEE ionic bonds form EVERY TIME there is a compound of a METAL AND A NONMETAL and NEVER in a compound of NON-METAL and a NONMETAL.
4. NEVER say that a nonpolar molecule is NOT ATTRACTED TO or (even worse!) REPELLED by a polar molecule! ALL MOLECULES ATTRACT EACH OTHER BUT polar molecules attract other polar molecules to a greater degree BECAUSE polar molecules have PERMANENT and relatively GREATER partial negative and partial positive regions to each molecule than do nonpolar molecules.
5. IF YOU BOTHER to take the time to draw a picture of what you are talking about, REFER TO YOUR DRAWING REPEATEDLY THROUGHOUT YOUR EXPLANATION!!! EVERY SINGLE TEST, TIME AFTER TIME, PEOPLE FORGET TO DO THIS AND, AS A RESULT, THEY LOSE CRAZY POINTS EVERY TIME!!!
6. DO NOT WRITE OUT YOUR ENTIRELY REHEARSED ANSWERS IF THEY ARE NOT RELATED TO THE QUESTION!!! NO WONDER YOU RUN OUT OF TIME!!! I cannot tell you how many students wrote paragraphs of irrelevant facts such as explaining that "solid salts do not conduct electricity" EVEN THOUGH SUCH A QUESTION DID NOT REMOTELY EXIST ON THE LAST TEST!
7. IF you do not yet know how to go from formula to name and vice-versa for any type of compound that we have covered so far, then you are going to lose A LOT of points on tomorrow's test. You cannot do math of chem or any kind of chemical equations unless you know how to name compounds and how to write formulas from names.
MAKE ABSOLUTELY SURE that you are bulletproof on naming or you are not going to do well.
AP: Microsoft Excel, WHO KNEW?
Why I haven't used Excel for graphing and linear regression is a mystery that one can only chalk up to senescence (the bad kind).
Y'all have Excel or you can use Google spreadsheets (free!) to do your kinetics dirty-work.
Here is the nice, easy, concise tutorial. I became adroit (mad skillz) within 40 minutes, well worth the time.
Data to Graph Setup
Linear Regression with Equation!
Y'all have Excel or you can use Google spreadsheets (free!) to do your kinetics dirty-work.
Here is the nice, easy, concise tutorial. I became adroit (mad skillz) within 40 minutes, well worth the time.
Data to Graph Setup
Linear Regression with Equation!
Monday, January 29, 2007
AP worksheet errata
thanks again to Mackey and Katie, who stayed until 6:45 PM and unearthed three worksheet errors; from the worksheets posted on 01/25/07, here they are:
Worksheet 5: the answer to 3b is 15.9 minutes, NOT 17.4 minutes
Worksheet 7: the answer to 2a is 1st order in A AND 2nd order in B; the real answer to 2b. is 150 M^-2 s^-1
Good turnout at extra help; that should mean good times tomorrow.
Worksheet 5: the answer to 3b is 15.9 minutes, NOT 17.4 minutes
Worksheet 7: the answer to 2a is 1st order in A AND 2nd order in B; the real answer to 2b. is 150 M^-2 s^-1
Good turnout at extra help; that should mean good times tomorrow.
Monday, Day 5
AP: we waded through the Spec-20 Absorbance to Concentration Data problem. Sorry I didn't anticipate the mental gymnastics required to make the data "work"; on the other hand, sometimes it is good to figure things out on the fly.
We finished kinetics by discussing how the Arrhenius equation relates the rate constant to the activation energy for a reaction; that is, the higher the activation energy for a given reaction, the LOWER the rate constant; HOWEVER, you CANNOT say that those variable are simply inversely proportional because they do not follow the equation xy=constant (or k x Ea = constant). Just say that, as one increases, the other decreases, good enough.
We then began our long journey into equilibrium by defining the term, noting that equilibrium NEVER EVER EVER means equal CONCENTRATIONS of reactants and products (never has been, never is, never will be); equilibrium CAN and MUST only pertain to equal RATES of forward and reverse processes or reactions.
We saw that, for varying initial concentrations of reactants and products, the equilibrium concentrations will ALSO vary. HOWEVER, there is a way to arrange the equilibrium concentration values in a certain ratio such that the ratio is a constant no matter what the initial reactant and product concentrations were (assuming the same Temperature for each experiment). We call that "arrangement" of the reactant and product concentrations, the EQUILIBRIUM CONSTANT EXPRESSION, which is the concentrations of the products to their respective coefficients (from the balanced equation) divided by the concentrations of the reactants to their respective coefficients.
Tomorrow's exam will cover the whole kinetics unit and will involve more calculations than explanations. There will also be a descriptive chem set of 5 questions with labeling of reaction type as well as balancing.
Honors: we balanced several equations and practiced how to balance equations that result in a non-integer coefficient for a reactant or product; we ended up just multiplying the entire equation by whatever number is in the denominator of the fraction (non-integer).
We then reviewed five types of chemical reactions by showing what they generally look like.
After that, we began calculations of gram-atomic mass, gram-molecular mass, and gram-formula mass of elements and compounds, respectively. These calculations are really the basis and heart of this unit so practice them on the posted worksheets.
There is a test on WEDNESDAY, covering the stated online objectives as well as chemical reaction types and balancing.
Regents: we finished our lab test review; corrections from that test are due on Thursday. I hope that you are now much more familiar and comfortable with that material and with your new seats (let me know if you have trouble seeing the screen from your new vantage point).
We then reviewed vapor pressure and normal boiling points relative to the strength of the intermolecular attractions. The STRONGER the intermolecular force of attraction, the LOWER the vapor pressure at a given temperature, and also the higher the normal boiling point of the substance.
We also reviewed electrolytes ( acids, bases, and soluble salts) and non-electrolytes (all molecules except for acids).
We will definitely finish the unit tomorrow and start on the Math of Chemistry. It is VERY important that you ALREADY know how to name compounds before we start this unit.
There is a test on THURSDAY covering the bonding unit objectives that were posted over the weekend. The test will emphasize intermolecular attractions and physical properties that are caused by the degree of intermolecular attractions.
We finished kinetics by discussing how the Arrhenius equation relates the rate constant to the activation energy for a reaction; that is, the higher the activation energy for a given reaction, the LOWER the rate constant; HOWEVER, you CANNOT say that those variable are simply inversely proportional because they do not follow the equation xy=constant (or k x Ea = constant). Just say that, as one increases, the other decreases, good enough.
We then began our long journey into equilibrium by defining the term, noting that equilibrium NEVER EVER EVER means equal CONCENTRATIONS of reactants and products (never has been, never is, never will be); equilibrium CAN and MUST only pertain to equal RATES of forward and reverse processes or reactions.
We saw that, for varying initial concentrations of reactants and products, the equilibrium concentrations will ALSO vary. HOWEVER, there is a way to arrange the equilibrium concentration values in a certain ratio such that the ratio is a constant no matter what the initial reactant and product concentrations were (assuming the same Temperature for each experiment). We call that "arrangement" of the reactant and product concentrations, the EQUILIBRIUM CONSTANT EXPRESSION, which is the concentrations of the products to their respective coefficients (from the balanced equation) divided by the concentrations of the reactants to their respective coefficients.
Tomorrow's exam will cover the whole kinetics unit and will involve more calculations than explanations. There will also be a descriptive chem set of 5 questions with labeling of reaction type as well as balancing.
Honors: we balanced several equations and practiced how to balance equations that result in a non-integer coefficient for a reactant or product; we ended up just multiplying the entire equation by whatever number is in the denominator of the fraction (non-integer).
We then reviewed five types of chemical reactions by showing what they generally look like.
After that, we began calculations of gram-atomic mass, gram-molecular mass, and gram-formula mass of elements and compounds, respectively. These calculations are really the basis and heart of this unit so practice them on the posted worksheets.
There is a test on WEDNESDAY, covering the stated online objectives as well as chemical reaction types and balancing.
Regents: we finished our lab test review; corrections from that test are due on Thursday. I hope that you are now much more familiar and comfortable with that material and with your new seats (let me know if you have trouble seeing the screen from your new vantage point).
We then reviewed vapor pressure and normal boiling points relative to the strength of the intermolecular attractions. The STRONGER the intermolecular force of attraction, the LOWER the vapor pressure at a given temperature, and also the higher the normal boiling point of the substance.
We also reviewed electrolytes ( acids, bases, and soluble salts) and non-electrolytes (all molecules except for acids).
We will definitely finish the unit tomorrow and start on the Math of Chemistry. It is VERY important that you ALREADY know how to name compounds before we start this unit.
There is a test on THURSDAY covering the bonding unit objectives that were posted over the weekend. The test will emphasize intermolecular attractions and physical properties that are caused by the degree of intermolecular attractions.
Friday, January 26, 2007
AP Chem: Ti-83, Ti-89 Linear Regression Tutorials
We did a kinetic rate law problem with the help of our Ti-83's today; on Monday, we will do an Arrhenius problem using the Ti calculators...
Ti-83 Tutorial
Ti-89 Tutorial
click on "best fit linear regression" on the menu on the right side of the page to see the tutorial with Ti-8x screenshots.
Here's another good tutorial with many relevant calculator functions with screenshots. This page is actually no longer on the original website but was saved by the almighty "archive.org" site, which saves the entire internet for your convenience.
Ti-83 Tutorial
Ti-83 Tutorial
Ti-89 Tutorial
click on "best fit linear regression" on the menu on the right side of the page to see the tutorial with Ti-8x screenshots.
Here's another good tutorial with many relevant calculator functions with screenshots. This page is actually no longer on the original website but was saved by the almighty "archive.org" site, which saves the entire internet for your convenience.
Ti-83 Tutorial
Friday, Day 4
Honors: we continued with the Math of Chem by focusing on the Law of Conservation of Mass and Charge as applied to "balancing chemical equations/reactions". I gave you several tips on how to balance the equations efficiently. Try to do the balancing worksheets this weekend (to be posted by Saturday) and I will give you some more examples and practice with that on Monday.
We also talked about some types of reactions (D Period, we only got to "double replacement", so you should look at the rest of the reaction types on page 4 of the "Math of Chem" notes that are now online). We will review them on Monday also.
Regents: we reviewed the second quarter lab exam; I tried to EMPHASIZE test-taking skills and how to AVOID errors on future tests by DRAWING and LABELING and PRE-PHRASING your answers before you look at the answer choices. We will finish that review on Monday. The previous test, Bonding II, corrections are due on Monday. Make sure that you KNOW why you got an answer wrong and WHY the correct answer is CORRECT. Email me if you have a problem understanding something.
AP: thanks to Mackey for showing us the ins and outs of the Ti calc. I will put up tutorials for the 83 and 89 and I will try to put up some more Kinetics problems that have worked out solutions from the raw experimental data via the calculator. I will also post the problems that we did today.
On Monday, we will solve some rate CONSTANT and activation energy problems by using graphical analysis and the Arrhenius equation.
We also talked about some types of reactions (D Period, we only got to "double replacement", so you should look at the rest of the reaction types on page 4 of the "Math of Chem" notes that are now online). We will review them on Monday also.
Regents: we reviewed the second quarter lab exam; I tried to EMPHASIZE test-taking skills and how to AVOID errors on future tests by DRAWING and LABELING and PRE-PHRASING your answers before you look at the answer choices. We will finish that review on Monday. The previous test, Bonding II, corrections are due on Monday. Make sure that you KNOW why you got an answer wrong and WHY the correct answer is CORRECT. Email me if you have a problem understanding something.
AP: thanks to Mackey for showing us the ins and outs of the Ti calc. I will put up tutorials for the 83 and 89 and I will try to put up some more Kinetics problems that have worked out solutions from the raw experimental data via the calculator. I will also post the problems that we did today.
On Monday, we will solve some rate CONSTANT and activation energy problems by using graphical analysis and the Arrhenius equation.
Thursday, January 25, 2007
AP: the catalyst and the rate constant
Occasionally, some interesting conundrums are posed in class; such an enigma was expressed today. I decided to "read the book" in order to clarify my answer and, though I found no explicit answers in any of the lofty tomes of chemistry, I can finally infer the correct answer to the problem presented today.
The question was: since catalysts LOWER the activation energy required for an effective collision, and the Arrhenius equation RELATES activation energy to the rate constant as follows: ln k = -Ea/RT + lnA, doesn't a catalyst increase the value of the rate constant?
I had to reply, "No", because of the unexplained DOGMA that ONLY TEMPERATURE can ever, ever, ever affect the rate constant for a reaction. That is, was, and always will be true, Now, however, I can clarify why a catalyst doesn't affect the rate constant (and here is the source of the conundrum) of the GIVEN reaction (the one WITHOUT the catalyst)!
Since a catalyst provides (say it together) "an alternative chemical pathway" for the same net reaction, the catalyst is IN THE RATE LAW of the ALTERNATE (catalyzed) reaction which has its own GREATER rate constant (compared to the SEPARATE and DISTINCT rate constant of the uncatalyzed reaction).
For example,
uncatalyzed reaction rate law: rate = k [A]
catalyzed reaction rate law: rate = k'[Q]{A] , where "Q" is the catalyst in the rate-determining step.
and k' is a greater number than k. So the catalyst DID NOT affect the ORIGINAL rate constant; the catalyzed reaction has its own different (and greater) rate constant.
In contrast, TEMPERATURE increases do NOT change the mechanism of a given reaction, so they do increase the SAME reaction's rate constant by increasing the number of effective collisions per second occurring in the reaction.
The end.
Now, I can sleep better.
The question was: since catalysts LOWER the activation energy required for an effective collision, and the Arrhenius equation RELATES activation energy to the rate constant as follows: ln k = -Ea/RT + lnA, doesn't a catalyst increase the value of the rate constant?
I had to reply, "No", because of the unexplained DOGMA that ONLY TEMPERATURE can ever, ever, ever affect the rate constant for a reaction. That is, was, and always will be true, Now, however, I can clarify why a catalyst doesn't affect the rate constant (and here is the source of the conundrum) of the GIVEN reaction (the one WITHOUT the catalyst)!
Since a catalyst provides (say it together) "an alternative chemical pathway" for the same net reaction, the catalyst is IN THE RATE LAW of the ALTERNATE (catalyzed) reaction which has its own GREATER rate constant (compared to the SEPARATE and DISTINCT rate constant of the uncatalyzed reaction).
For example,
uncatalyzed reaction rate law: rate = k [A]
catalyzed reaction rate law: rate = k'[Q]{A] , where "Q" is the catalyst in the rate-determining step.
and k' is a greater number than k. So the catalyst DID NOT affect the ORIGINAL rate constant; the catalyzed reaction has its own different (and greater) rate constant.
In contrast, TEMPERATURE increases do NOT change the mechanism of a given reaction, so they do increase the SAME reaction's rate constant by increasing the number of effective collisions per second occurring in the reaction.
The end.
Now, I can sleep better.
Thursday, Day 3
AP: we discussed the Arrhenius equation including the meaning and relevance of each factor; we showed the relationships among the variables and saw how the equation, since it is governed by the distribution of molecular speeds at a given temperature, has the same form as the Claussius-Clayperon equation, which is also governed by the same factor.
We then applied the equation in several situations.
Tomorrow, we will do some final examples and also see a couple of examples of the two types of catalyst.
I will emphasize how graphical data relates to what is occurring in a given problem.
Regents: we related the type of intermolecular forces of attraction to the vapor pressure of a given substance; we then applied this information to Table H and related vapor pressure to boiling point and "normal" boiling point. Tomorrow, we will finish the unit on IMFA.
Honors: we finished the Bonding Unit by discussing IMFA, vapor pressure, and boiling points of various types of substances. We then focused on the various types of solids and their respective melting points. The new substance types discussed were "network (covalent) solids" and polymers. Network solids are unique in that they are a lattice of either C or Si (or both) atoms that are ALL covalently bonded, four atoms to each central atom.
We then FINALLY began the Math of Chem unit by discussing the parts of a chemical equation and how the Law of Conservation of Mass and Charge MUST be obeyed in any chemical equation.
We then applied the equation in several situations.
Tomorrow, we will do some final examples and also see a couple of examples of the two types of catalyst.
I will emphasize how graphical data relates to what is occurring in a given problem.
Regents: we related the type of intermolecular forces of attraction to the vapor pressure of a given substance; we then applied this information to Table H and related vapor pressure to boiling point and "normal" boiling point. Tomorrow, we will finish the unit on IMFA.
Honors: we finished the Bonding Unit by discussing IMFA, vapor pressure, and boiling points of various types of substances. We then focused on the various types of solids and their respective melting points. The new substance types discussed were "network (covalent) solids" and polymers. Network solids are unique in that they are a lattice of either C or Si (or both) atoms that are ALL covalently bonded, four atoms to each central atom.
We then FINALLY began the Math of Chem unit by discussing the parts of a chemical equation and how the Law of Conservation of Mass and Charge MUST be obeyed in any chemical equation.
Wednesday, January 24, 2007
Wednesday, Day 2
Honors: we discussed the physical properties of vapor pressure and boiling point as related to intermolecular forces of attraction. The STRONGER the intermolecular forces of attraction (IMFA), the lower the fraction of molecules that can overcome their intermolecular attractions at a given temperature (avg. KE of molecules in sample), therefore the lower the vapor pressure. Also, the STRONGER the IMFA, the higher the temperature required to get the substance to have a vapor pressure equal to that of the opposing atmospheric pressure, therefore, the higher the boiling point (temperature) of the substance.
Tomorrow, we will finish our discussion of IMFA as related to melting points and solubility; then we will briefly discuss/review the types of solids and attractions OR bonds within the solid lattice of particles. We then MUST begin the math of chem unit, so I will at least give you a brief introduction to how to relate various quantities in a chemical reaction/equation.
Regents: we focused on intermolecular forces of attraction (IMFA) and gave examples of substances (and, for some, mixtures) that exhibit a given type of IMFA. It is important to be able to know, just by drawing out the Lewis structure of a given molecule, what type of IMFA that the molecule has and also how relatively strong that IMFA is. We will further discuss how the IMFA's relate to the various physical properties of substances.
AP: we finished deriving the time-dependent rate law and the the half-life equation for a second order reaction. We then did the same for a zeroth order reaction. We then did a few examples involving the second order rate equation.
Note: the raw data of concentration vs. time will not yield constant "half-life" time intervals for a second order reaction; only first order reactions half constant half-lives that are independent of reactant concentration.
It is important to relate the equations to the expected graphs, to know what the slopes indicate, and to be able to calculate the rate constant, WITH UNITS, from the graphs.
We talked a bit about collision theory, which will lead to the Arrhenius equation that relates a rate constant to the activation energy requirement for a given reaction.
NOTE: I did misspeak at the very end of class; I SHOULD HAVE SAID that the Arrhenius equation shows that THE LOWER the activation energy, THE HIGHER (faster) the rate constant (that is accounted for by the negative sign in the equation:
k= A e^(-Ea/RT)... also, from the last test, I noticed that not everyone uses a small (lowercase) k for the rate constant; that is VERY BAD because BIG K is RESERVED for the "equilibrium constant" ONLY. Be careful with that.
Thanks.
p.s. I don't think you all know how good you are at descriptive chem; on the last test, all but one of you got almost ALL of them right!
With a little more experience, question 4 may net you the easiest 15 points on the AP exam in the shortest amount of time. Consider that the national AVERAGE score on that question is 5 out of 15 !!!
Tomorrow, we will finish our discussion of IMFA as related to melting points and solubility; then we will briefly discuss/review the types of solids and attractions OR bonds within the solid lattice of particles. We then MUST begin the math of chem unit, so I will at least give you a brief introduction to how to relate various quantities in a chemical reaction/equation.
Regents: we focused on intermolecular forces of attraction (IMFA) and gave examples of substances (and, for some, mixtures) that exhibit a given type of IMFA. It is important to be able to know, just by drawing out the Lewis structure of a given molecule, what type of IMFA that the molecule has and also how relatively strong that IMFA is. We will further discuss how the IMFA's relate to the various physical properties of substances.
AP: we finished deriving the time-dependent rate law and the the half-life equation for a second order reaction. We then did the same for a zeroth order reaction. We then did a few examples involving the second order rate equation.
Note: the raw data of concentration vs. time will not yield constant "half-life" time intervals for a second order reaction; only first order reactions half constant half-lives that are independent of reactant concentration.
It is important to relate the equations to the expected graphs, to know what the slopes indicate, and to be able to calculate the rate constant, WITH UNITS, from the graphs.
We talked a bit about collision theory, which will lead to the Arrhenius equation that relates a rate constant to the activation energy requirement for a given reaction.
NOTE: I did misspeak at the very end of class; I SHOULD HAVE SAID that the Arrhenius equation shows that THE LOWER the activation energy, THE HIGHER (faster) the rate constant (that is accounted for by the negative sign in the equation:
k= A e^(-Ea/RT)... also, from the last test, I noticed that not everyone uses a small (lowercase) k for the rate constant; that is VERY BAD because BIG K is RESERVED for the "equilibrium constant" ONLY. Be careful with that.
Thanks.
p.s. I don't think you all know how good you are at descriptive chem; on the last test, all but one of you got almost ALL of them right!
With a little more experience, question 4 may net you the easiest 15 points on the AP exam in the shortest amount of time. Consider that the national AVERAGE score on that question is 5 out of 15 !!!
Tuesday, January 23, 2007
Tuesday, Day 1: THIRD QUARTER COMMENCES!
AP: we learned the time-dependent expressions of the rate laws for first and second order reactions. From the first order equation, we determined the relationship half-life time and the rate constant of a first order reaction (remember, you only need to know the UNITS of the rate constant to know the order of the reaction).
We will derive the second order half-life equation tomorrow as well as the zeroth order rate law, constant, and half-life equation....good math, good times...thanks for the help with the calculus today!
Honors: today we reviewed the various types of intermolecular attractions; we began to relate the relative strengths of these intermolecular attractions to various physical properties: vapor pressure, boiling point temperature, melting point temperature, and miscibility/solubility with other substances.
We will continue these explanations tomorrow.
Regents: we reviewed the intermolecular forces of attraction and gave multiple examples of molecules that exhibit a given type of attraction e.g. H2O = hydrogen "bonding" attractions , Br2 = induced dipole/London Dispersion/VanderWaal's attractions.
We mostly focused on induced dipole attractions. Tomorrow, we will relate each type of intermolecular attraction to the physical properties of the substances.
Study your notes...
We will derive the second order half-life equation tomorrow as well as the zeroth order rate law, constant, and half-life equation....good math, good times...thanks for the help with the calculus today!
Honors: today we reviewed the various types of intermolecular attractions; we began to relate the relative strengths of these intermolecular attractions to various physical properties: vapor pressure, boiling point temperature, melting point temperature, and miscibility/solubility with other substances.
We will continue these explanations tomorrow.
Regents: we reviewed the intermolecular forces of attraction and gave multiple examples of molecules that exhibit a given type of attraction e.g. H2O = hydrogen "bonding" attractions , Br2 = induced dipole/London Dispersion/VanderWaal's attractions.
We mostly focused on induced dipole attractions. Tomorrow, we will relate each type of intermolecular attraction to the physical properties of the substances.
Study your notes...
Monday, January 22, 2007
Monday, Day 7 (UPDATED with test results...)
EVERYBODY LISTEN UP!: There ARE lab questions ON THE REGENTS EXAM!!!
Honors: we had our Lab test today, results within the hour...
UPDATE: Both classes did considerably worse than the past two years' classes! Again, NOBODY at extra help on Friday after rah-rah pep rally shows how another missed opportunity for preparation that is sorely needed will continue to result in an abundance of errors. These errors were made on MULTIPLE CHOICE questions that were ALL DIRECTLY AND SOLELY FROM THE OBJECTIVES, which were based on problems that we have repeatedly done in CLASS.
WHAT A COINCIDENCE!!!! The THREE students who obviously prepared and then, on Saturday, emailed me their work for clarification all scored above a 96!!!
D Period: 80
G Period: 78
Way to end the quarter.
tomorrow, we continute with intermolecular attractions and their effect on physical properties. All of the explanations that we have been doing and the explanations that we are about to cover will be on the bonding/attractions written unit test next week; take excellent notes and stay focused in class.
Regents:
more on intermolecular attractions and their relationship to melting/boiling points and solubility in the next few lessons.
we did our Lab test today; results soon...
UPDATE: the results are in. I should have know from the ghost-town of extra help last week that the class was not preparing.
Expect a true failing grade (below 65) if you were anywhere near failure before this test. The grades on most tests were atrocious; clearly the result of a total lack of preparation/practice and any kind of note-taking in class!!! The fact that , EVERY DAY, I have to tell many of you to take notes in class demonstrates the lack of maturity and seriousness of many in this class! The fact that I can barely get many of you to EVEN LOOK AT THE BLACKBOARD OR SMARTBOARD, unless I am BURNING something, demonstrates that many of you have a severe problem that undermines and retards your academic ability! That problem is LAZINESS and WANTON ignorance and, if you do not rid yourself of those flaws, you will fail, plain and simple. A full ONE-THIRD of the class didn't even do a simple assignment of copying bonding-term definitions.
You will definitely fail the rest of the year and the course if this pathetic lack of preparation continues!
Be ready to work tomorrow!!!
AP: we did more rate law problems from data that wasn't so cookie-cutter-nice via the ratio method and saw that you ultimately get two equations with two unknowns that you just combine via division to make one of the variables drop out. You then solve for the exponent of one reactant in the rate law. Then, plugging in that found exponent, you solve for the other one (assuming two reactants in the rate law).
We then saw that rate laws can actually have negative exponents; rate laws can also have fractional exponents. These can still be shown to be related to the slow/rate determining step usually via a fast equilibrium step just before the rate determining step.
We then finished the typical question series in a problem: solve for k, with units of course; solve for the rate of reaction in a different experiment at the same temperature with different concentrations of reactants.
Tomorrow, we launch into the all-powerful "time-dependent" rate law equations for zeroth, first, and second order reactions!
Honors: we had our Lab test today, results within the hour...
UPDATE: Both classes did considerably worse than the past two years' classes! Again, NOBODY at extra help on Friday after rah-rah pep rally shows how another missed opportunity for preparation that is sorely needed will continue to result in an abundance of errors. These errors were made on MULTIPLE CHOICE questions that were ALL DIRECTLY AND SOLELY FROM THE OBJECTIVES, which were based on problems that we have repeatedly done in CLASS.
WHAT A COINCIDENCE!!!! The THREE students who obviously prepared and then, on Saturday, emailed me their work for clarification all scored above a 96!!!
D Period: 80
G Period: 78
Way to end the quarter.
tomorrow, we continute with intermolecular attractions and their effect on physical properties. All of the explanations that we have been doing and the explanations that we are about to cover will be on the bonding/attractions written unit test next week; take excellent notes and stay focused in class.
Regents:
more on intermolecular attractions and their relationship to melting/boiling points and solubility in the next few lessons.
we did our Lab test today; results soon...
UPDATE: the results are in. I should have know from the ghost-town of extra help last week that the class was not preparing.
Expect a true failing grade (below 65) if you were anywhere near failure before this test. The grades on most tests were atrocious; clearly the result of a total lack of preparation/practice and any kind of note-taking in class!!! The fact that , EVERY DAY, I have to tell many of you to take notes in class demonstrates the lack of maturity and seriousness of many in this class! The fact that I can barely get many of you to EVEN LOOK AT THE BLACKBOARD OR SMARTBOARD, unless I am BURNING something, demonstrates that many of you have a severe problem that undermines and retards your academic ability! That problem is LAZINESS and WANTON ignorance and, if you do not rid yourself of those flaws, you will fail, plain and simple. A full ONE-THIRD of the class didn't even do a simple assignment of copying bonding-term definitions.
You will definitely fail the rest of the year and the course if this pathetic lack of preparation continues!
Be ready to work tomorrow!!!
AP: we did more rate law problems from data that wasn't so cookie-cutter-nice via the ratio method and saw that you ultimately get two equations with two unknowns that you just combine via division to make one of the variables drop out. You then solve for the exponent of one reactant in the rate law. Then, plugging in that found exponent, you solve for the other one (assuming two reactants in the rate law).
We then saw that rate laws can actually have negative exponents; rate laws can also have fractional exponents. These can still be shown to be related to the slow/rate determining step usually via a fast equilibrium step just before the rate determining step.
We then finished the typical question series in a problem: solve for k, with units of course; solve for the rate of reaction in a different experiment at the same temperature with different concentrations of reactants.
Tomorrow, we launch into the all-powerful "time-dependent" rate law equations for zeroth, first, and second order reactions!
Sunday, January 21, 2007
MISinformation
Though I generally do not find on the internet such horrifyingly bad information regarding simple scientific definitions, I did google some terms that were asked by your classmates and found the information to be misleading and dumb.
Here are two definitions that were given in class but that I will reiterate for you now:
COHESION vs. ADHESION:
Cohesion means attraction between and among the molecules of ONE SINGLE SUBSTANCE e.g. water molecules attracting water molecules via EXTREME dipole-dipole attractions/hydrogen-bonding-ATTRACTIONS.
Here is an image of water droplets "beading up" on a less polar substance's surface. The water molecules attract each other more than they are attracted to the molecules that make up the surface, thus, the water molecules cling together and form droplets:

Adhesion means attraction between and among the molecules/particles of TWO DIFFERENT SUBSTANCES e.g. water adheres to glass.
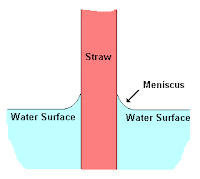
Here is an image and a diagram showing the adhesion of water molecules to the surface of a plastic straw; the plastic is made of some generally nonpolar polymer molecules:

Here are two definitions that were given in class but that I will reiterate for you now:
COHESION vs. ADHESION:
Cohesion means attraction between and among the molecules of ONE SINGLE SUBSTANCE e.g. water molecules attracting water molecules via EXTREME dipole-dipole attractions/hydrogen-bonding-ATTRACTIONS.
Here is an image of water droplets "beading up" on a less polar substance's surface. The water molecules attract each other more than they are attracted to the molecules that make up the surface, thus, the water molecules cling together and form droplets:

Adhesion means attraction between and among the molecules/particles of TWO DIFFERENT SUBSTANCES e.g. water adheres to glass.
Here is an image and a diagram showing the adhesion of water molecules to the surface of a plastic straw; the plastic is made of some generally nonpolar polymer molecules:


Friday, January 19, 2007
Pep Rally Day 6
AP: Over the weekend, I will grade the test from today. Check out these tutorials to give yourself a better understanding of Kinetics, which we should be able to finish next week:
Kinetics Tutorial
Kinetics Videos and Animations
Regents: today, we covered the remaining types of intermolecular attractions and how to recognize which molecules have which attractions. Overall, you now know ion-dipole, hydrogen "bonding", dipole-dipole, and induced dipole attractions. Check out the tutorial (from the class website) regarding those attractions:
Intermolecular attractions tutorial
Monday, we have a lab exam, which will be the final 100 points for the quarter. The objectives for that exam are posted on the website. Make SURE that you know everything about them intuitively, by Monday. If you have a question about a particular objective, just email me before Sunday afternoon/early evening. Don't forget about the GRADED hw assignment that you must hand in by Monday, if you have not already done so: Orange Review Book Topic 6 Bonding vocabulary word definitions, complete and thorough. Those points will help your second quarter grade.
Honors: today, we covered the remaining types of intermolecular attractions and how to recognize which molecules have which attractions. Overall, you now know ion-dipole, hydrogen "bonding", dipole-dipole, and induced dipole attractions. Check out the tutorial (from the class website) regarding those attractions:
Intermolecular attractions tutorial
Monday, we have a lab exam, which will be the final 100 points for the quarter. The objectives for that exam are posted on the website. Make SURE that you know everything about them intuitively, by Monday. If you have a question about a particular objective, just email me before Sunday afternoon/early evening.
I intend to finish the Bonding Unit by next Wednesday after which we start the Math of Chemistry unit! For approximately the next 6 weeks, the course will mostly involve relationships/scientific laws/equations that involve division, multiplication, and ratios. All formulas will have to be repeatedly practiced and applied until you know them by heart because you will not be given any equations/formulas on any test.
FAIR WARNING: In the equations and calculations, all measurements and constants that have physical units (ex: grams or moles or liters) must be written WITH those units or you will not receive ANY credit for your answers. Proper cancellation of units in calculations is also required. Do not EVER forget those two requirements or your third quarter will be a disaster for sure. Do not ever practice taking shortcuts without units or you will be training yourself to fail.
On the other hand, you won't be asked to "explain" an equation; you will just have to know HOW and WHEN to apply the equation. We have been through two quarters so, even if you still have not yet been able to write/draw a good explanation (I don't know how that is possible by now, though), you, at least, now know what is expected when you are asked to explain something.
In the past, the majority of students start to achieve higher grades in the third quarter because they take the Math of Chem section very seriously and practice each problem type until they know it inside out. The problems are very direct and do not have too much variety; each can be mastered consecutively and even combined in a several step problem.
Study hard for Monday.
Kinetics Tutorial
Kinetics Videos and Animations
Regents: today, we covered the remaining types of intermolecular attractions and how to recognize which molecules have which attractions. Overall, you now know ion-dipole, hydrogen "bonding", dipole-dipole, and induced dipole attractions. Check out the tutorial (from the class website) regarding those attractions:
Intermolecular attractions tutorial
Monday, we have a lab exam, which will be the final 100 points for the quarter. The objectives for that exam are posted on the website. Make SURE that you know everything about them intuitively, by Monday. If you have a question about a particular objective, just email me before Sunday afternoon/early evening. Don't forget about the GRADED hw assignment that you must hand in by Monday, if you have not already done so: Orange Review Book Topic 6 Bonding vocabulary word definitions, complete and thorough. Those points will help your second quarter grade.
Honors: today, we covered the remaining types of intermolecular attractions and how to recognize which molecules have which attractions. Overall, you now know ion-dipole, hydrogen "bonding", dipole-dipole, and induced dipole attractions. Check out the tutorial (from the class website) regarding those attractions:
Intermolecular attractions tutorial
Monday, we have a lab exam, which will be the final 100 points for the quarter. The objectives for that exam are posted on the website. Make SURE that you know everything about them intuitively, by Monday. If you have a question about a particular objective, just email me before Sunday afternoon/early evening.
I intend to finish the Bonding Unit by next Wednesday after which we start the Math of Chemistry unit! For approximately the next 6 weeks, the course will mostly involve relationships/scientific laws/equations that involve division, multiplication, and ratios. All formulas will have to be repeatedly practiced and applied until you know them by heart because you will not be given any equations/formulas on any test.
FAIR WARNING: In the equations and calculations, all measurements and constants that have physical units (ex: grams or moles or liters) must be written WITH those units or you will not receive ANY credit for your answers. Proper cancellation of units in calculations is also required. Do not EVER forget those two requirements or your third quarter will be a disaster for sure. Do not ever practice taking shortcuts without units or you will be training yourself to fail.
On the other hand, you won't be asked to "explain" an equation; you will just have to know HOW and WHEN to apply the equation. We have been through two quarters so, even if you still have not yet been able to write/draw a good explanation (I don't know how that is possible by now, though), you, at least, now know what is expected when you are asked to explain something.
In the past, the majority of students start to achieve higher grades in the third quarter because they take the Math of Chem section very seriously and practice each problem type until they know it inside out. The problems are very direct and do not have too much variety; each can be mastered consecutively and even combined in a several step problem.
Study hard for Monday.
Thursday, January 18, 2007
Thursday, Day 5
AP: we got to the very heart of the unit today: we related a proposed reaction mechanism to the EXPERIMENTALLY DETERMINED rate law; by assigning the correct SLOW/RATE DETERMINING elementary step, we are able to see whether the rate law of the proposed mechanism is consistent with the EXPERIMENTALLY DETERMINED rate law.
We then showed both intuitively and mathematically how to determine the exponent on each reactant in a rate law expression.
This is an extremely important skill to develop and will definitely be a part II question on this year's AP exam (this skill is also tested on the Chem SAT II).
Tomorrow's test will involve everything that we covered in Kinetics so far as well as the unit on intermolecular forces especially the material that we covered since the last exam.
I will post the relevant kinetics hw on EDLINE.COM for now. Read relevant text Ch. 14 sections and do even # questions 14.40-14.48.
I won't be home until later to post the hw on the regular class webpage.
You must be logged in to edline in order for this link to work:
https://www.edline.net/files/40b81ca812a335d23745a49013852ec4/012405aphwa.pdf
Regents: we discussed induced dipole/ Van der Waal's/London dispersion attractions among NONPOLAR molecules. We drew pictures of how and why these dipoles are induced and how they create these relatively weak induced dipole attractions.
I will hand back your bonding exams tomorrow; corrections from that exam are due on Monday.
Honors: we had our Bonding Unit multiple choice exam today; early results look pretty good given that this unit test is actually more difficult than other unit tests because the subject matter depends on so much past knowledge from prior units.
Both periods have an average of about 90. That must mean that many of you are improving in your compound NAMING skills. GOOD times.
BOTH D and G periods must write FULL, NEAT, AND COMPLETE corrections for the last written-response bonding exam. Those corrections are due on Monday as grades will be entered on Monday night.
We then showed both intuitively and mathematically how to determine the exponent on each reactant in a rate law expression.
This is an extremely important skill to develop and will definitely be a part II question on this year's AP exam (this skill is also tested on the Chem SAT II).
Tomorrow's test will involve everything that we covered in Kinetics so far as well as the unit on intermolecular forces especially the material that we covered since the last exam.
I will post the relevant kinetics hw on EDLINE.COM for now. Read relevant text Ch. 14 sections and do even # questions 14.40-14.48.
I won't be home until later to post the hw on the regular class webpage.
You must be logged in to edline in order for this link to work:
https://www.edline.net/files/40b81ca812a335d23745a49013852ec4/012405aphwa.pdf
Regents: we discussed induced dipole/ Van der Waal's/London dispersion attractions among NONPOLAR molecules. We drew pictures of how and why these dipoles are induced and how they create these relatively weak induced dipole attractions.
I will hand back your bonding exams tomorrow; corrections from that exam are due on Monday.
Honors: we had our Bonding Unit multiple choice exam today; early results look pretty good given that this unit test is actually more difficult than other unit tests because the subject matter depends on so much past knowledge from prior units.
Both periods have an average of about 90. That must mean that many of you are improving in your compound NAMING skills. GOOD times.
BOTH D and G periods must write FULL, NEAT, AND COMPLETE corrections for the last written-response bonding exam. Those corrections are due on Monday as grades will be entered on Monday night.
Wednesday, January 17, 2007
Wednesday, Day 4
Honors: MULTIPLE CHOICE bonding unit test tomorrow, which will cover the unit on bonding, naming, and attractions up to and including "induced dipole' attractions that we covered in class today.
This correction came up at extra help:
on the file from 011407 entitled "Chemical Bonding Worksheets and Practice Tests", on the Bonding I Self Test,
Question #1 has two correct answers: during ion formation, the atom that TRANSFERS (loses) its valence electron(s) is the (metal atom) one with the LOWER electronegativity OR the LOWER ionization energy; both of those answers (B) and (C) are correct! To see this, just think of sodium chloride formation.
Question #20 is NOT written correctly; it SHOULD read, "ALL COVALENT chemical bonds are the result of ..."
the correct answer is then (D), "simultaneous attraction of electrons to two nuclei"; as an example, think of the covalent bond in an H2 molecule or in a CH4 molecule.
Today we reviewed ion-dipole attractions and their relationship to the solubility of salts. We then discussed ion-induced dipole attractions between a salt and a nonpolar solvent: these attractions are not strong enough to overcome ionic bonds, therefore salts are not soluble in nonpolar solvents.
We then explained the process of induced dipole formation as the source of attraction between/among nonpolar molecules. The more polarizable the molecule, due to a greater number of total electrons per molecule, the stronger and more frequent the induced dipoles and therefore, the greater the induced dipole attractions, which are sometimes called "London dispersion" or "Van der Waal's" attractions.
Regents: we reviewed parts of our last test and found a few more points for the class, good times. We then explained ion-dipole attractions and their relationship to the solubility of salts. When there are enough ion-dipole attractions between an ion and its surrounding polar solvent molecules, then the NET force of attraction between these particles may be sufficient to break the ionic bond between the ion and surrounding oppositely charged ions so that the ion (salt) will dissolve/be solvated/be hydrated.
AP: today, we reviewed the various graphs that yield 0th, 1st, 2nd, and 3rd order rate constants and the units of each.
Then we explained , statistically, how the exponent of a rate law MUST match the molecularity of the elementary step.
We then showed that the SLOW step of a reaction is the "rate determining step".
We stated the criteria for a PLAUSIBLE proposed reaction mechanism: how the rate determining step of the proposed mechanism MUST agree with the EXPERIMENTALLY DETERMINED rate law ( there is NO OTHER WAY to get a rate law for a reaction!). Also, the sum of the mechanism steps must yield the overall balance equation.
We finished by showing how some mechanisms can agree with or not concur with an experimentally determined rate law. We will do more of these problems tomorrow.
This correction came up at extra help:
on the file from 011407 entitled "Chemical Bonding Worksheets and Practice Tests", on the Bonding I Self Test,
Question #1 has two correct answers: during ion formation, the atom that TRANSFERS (loses) its valence electron(s) is the (metal atom) one with the LOWER electronegativity OR the LOWER ionization energy; both of those answers (B) and (C) are correct! To see this, just think of sodium chloride formation.
Question #20 is NOT written correctly; it SHOULD read, "ALL COVALENT chemical bonds are the result of ..."
the correct answer is then (D), "simultaneous attraction of electrons to two nuclei"; as an example, think of the covalent bond in an H2 molecule or in a CH4 molecule.
Today we reviewed ion-dipole attractions and their relationship to the solubility of salts. We then discussed ion-induced dipole attractions between a salt and a nonpolar solvent: these attractions are not strong enough to overcome ionic bonds, therefore salts are not soluble in nonpolar solvents.
We then explained the process of induced dipole formation as the source of attraction between/among nonpolar molecules. The more polarizable the molecule, due to a greater number of total electrons per molecule, the stronger and more frequent the induced dipoles and therefore, the greater the induced dipole attractions, which are sometimes called "London dispersion" or "Van der Waal's" attractions.
Regents: we reviewed parts of our last test and found a few more points for the class, good times. We then explained ion-dipole attractions and their relationship to the solubility of salts. When there are enough ion-dipole attractions between an ion and its surrounding polar solvent molecules, then the NET force of attraction between these particles may be sufficient to break the ionic bond between the ion and surrounding oppositely charged ions so that the ion (salt) will dissolve/be solvated/be hydrated.
AP: today, we reviewed the various graphs that yield 0th, 1st, 2nd, and 3rd order rate constants and the units of each.
Then we explained , statistically, how the exponent of a rate law MUST match the molecularity of the elementary step.
We then showed that the SLOW step of a reaction is the "rate determining step".
We stated the criteria for a PLAUSIBLE proposed reaction mechanism: how the rate determining step of the proposed mechanism MUST agree with the EXPERIMENTALLY DETERMINED rate law ( there is NO OTHER WAY to get a rate law for a reaction!). Also, the sum of the mechanism steps must yield the overall balance equation.
We finished by showing how some mechanisms can agree with or not concur with an experimentally determined rate law. We will do more of these problems tomorrow.
Tuesday, January 16, 2007
Tuesday, Day 3
Enjoy the last day of warmth...the rest of this week will be very cold...
AP: we went through a litany of definitions and equations today: relating rates of appearance of reactants to rates of disappearance of products to the rate of reaction, which is really the rate "per mole of reaction". We discussed elementary steps of a proposed reaction mechanism, the molecularity and order of each step, the initial requirement for plausibility of a proposed mechanism (the sum of the elementary steps MUST yield the balanced overall stoichiometric equation). We introduced RATE LAW, which is "rate= k[A]^x[B]^y..." and RATE CONSTANT, which is the "k" in the rate law equation.
These terms and equations will make sense ONLY if you practice and apply them regularly so that you know how, when, and where to use them! Of course, we will be doing many examples in class for the rest of the week. On Friday, as part of our test, we will have a section on descriptive chemistry and naming metal-ligand complex.
Honors: we reviewed the explanation for the relatively high melting points of salts/ionic compounds. We then explained both the nonconductivity of solid ionic compounds/salts and the excellent conductivity of molten ionic compounds in terms of ion and electron net mobility or lack thereof.
We started to explain the first of several types of attractions, ion-dipole attractions, which account for the dissolving of salts in POLAR molecular solvents such as water; these attractions are NOT bonds because they are much weaker per attraction than any type of covalent, ionic, or metallic bond. I will post the videos/animations from today's lesson when I get home.
Regents: we reviewed the explanation for the relatively high melting points of salts/ionic compounds. We then explained both the nonconductivity of solid ionic compounds/salts and the excellent conductivity of molten ionic compounds in terms of ion and electron net mobility or lack thereof.
For additional points towards your quarterly average, here is the next written homework to be handed in on Friday:
Orange Review Book Topic 6: Bonding
Clearly and completely define each of the terms in the unit Vocabulary List on page 79.
Hand that in with the complete and accurate corrections from your last test, which I will return to you later this week.
AP: we went through a litany of definitions and equations today: relating rates of appearance of reactants to rates of disappearance of products to the rate of reaction, which is really the rate "per mole of reaction". We discussed elementary steps of a proposed reaction mechanism, the molecularity and order of each step, the initial requirement for plausibility of a proposed mechanism (the sum of the elementary steps MUST yield the balanced overall stoichiometric equation). We introduced RATE LAW, which is "rate= k[A]^x[B]^y..." and RATE CONSTANT, which is the "k" in the rate law equation.
These terms and equations will make sense ONLY if you practice and apply them regularly so that you know how, when, and where to use them! Of course, we will be doing many examples in class for the rest of the week. On Friday, as part of our test, we will have a section on descriptive chemistry and naming metal-ligand complex.
Honors: we reviewed the explanation for the relatively high melting points of salts/ionic compounds. We then explained both the nonconductivity of solid ionic compounds/salts and the excellent conductivity of molten ionic compounds in terms of ion and electron net mobility or lack thereof.
We started to explain the first of several types of attractions, ion-dipole attractions, which account for the dissolving of salts in POLAR molecular solvents such as water; these attractions are NOT bonds because they are much weaker per attraction than any type of covalent, ionic, or metallic bond. I will post the videos/animations from today's lesson when I get home.
Regents: we reviewed the explanation for the relatively high melting points of salts/ionic compounds. We then explained both the nonconductivity of solid ionic compounds/salts and the excellent conductivity of molten ionic compounds in terms of ion and electron net mobility or lack thereof.
For additional points towards your quarterly average, here is the next written homework to be handed in on Friday:
Orange Review Book Topic 6: Bonding
Clearly and completely define each of the terms in the unit Vocabulary List on page 79.
Hand that in with the complete and accurate corrections from your last test, which I will return to you later this week.
Saturday, January 13, 2007
Regents and Honors Labs
I posted the Molecular Models lab, which is due Friday, January 19th. The molecular model kits are available at extra help if you need to work with them some more.
Both Regents and Honors classes will have a lab exam on Monday, January 22nd. That test will be graded immediately (seconds before grades are due) and will count towards your second quarter grade. The exam will cover all labs from this past quarter up to and including the electrolyte (light bulb conductivity tester) lab as well as standard lab questions regarding lab equipment, techniques, and also sig figs in calculations.
There are plenty of extra practice test and tutorial files just posted on our class website. Do those over the course of this week.
Both Regents and Honors classes will have a lab exam on Monday, January 22nd. That test will be graded immediately (seconds before grades are due) and will count towards your second quarter grade. The exam will cover all labs from this past quarter up to and including the electrolyte (light bulb conductivity tester) lab as well as standard lab questions regarding lab equipment, techniques, and also sig figs in calculations.
There are plenty of extra practice test and tutorial files just posted on our class website. Do those over the course of this week.
Friday, January 12, 2007
AP Descriptive Chemistry Practice
I have posted sets of AP Descriptive Chemistry questions from actual AP exams from 1981 through 2004. To actually benefit and augment your real chemistry knowledge from these sets, try to categorize each reaction by type and also balance the equations because those skills are now required on this question (which will be part II, question #4).
Here is another descriptive chem site, which will help us reach that perfect 15 out of 15 ( without taking up more than 5 minutes! ) on "Question 4" of the AP Chem exam, this excellent "teaching" site will give you countless practice problems.
http://dwb2.unl.edu/apchem/main.html
Just create a free account and then you can practice at will and you can get instant feedback.
The good thing about descriptive chemistry is that, once it "clicks" and you can "see" the products that will form from any of the eight common chem. reaction types, you and others will think that you have magical powers as you spout off sound bites such as, "...(chortle) of course, double replacement with precipitation...solid magnesium phosphate forms from those reactants" or "...child's play, an obvious metal-ligand complex reaction forming tetra-ammine copper II ion complex; note the number of ligands is twice the transition metal's charge...tee-hee!" or "Why are you walking away from me? I can't quit you!"
:)
Here is another descriptive chem site, which will help us reach that perfect 15 out of 15 ( without taking up more than 5 minutes! ) on "Question 4" of the AP Chem exam, this excellent "teaching" site will give you countless practice problems.
http://dwb2.unl.edu/apchem/main.html
Just create a free account and then you can practice at will and you can get instant feedback.
The good thing about descriptive chemistry is that, once it "clicks" and you can "see" the products that will form from any of the eight common chem. reaction types, you and others will think that you have magical powers as you spout off sound bites such as, "...(chortle) of course, double replacement with precipitation...solid magnesium phosphate forms from those reactants" or "...child's play, an obvious metal-ligand complex reaction forming tetra-ammine copper II ion complex; note the number of ligands is twice the transition metal's charge...tee-hee!" or "Why are you walking away from me? I can't quit you!"
:)
Friday, Day 2
Regents/Honors: the molecular model lab writeup will be posted this weekend and the lab is due later next week. That is the last lab that will be covered on our quarterly lab exam, which is the last grade for the second quarter.
Honors: we began the second half of the bonding unit in which we make the DISTINCTION between a true BOND and a mere intermolecular "attraction". There are three and ONLY three true types of BONDS: (1.) ionic (2.) covalent (3.) metallic .
A MUCH weaker form of attraction exists in certain cases: (1.) ion-dipole attractions (as in aqueous solutions of ions) (2.) dipole-dipole attractions among/between polar molecules; there is an extreme form of this attraction UNFORTUNATELY named "hydrogen bonding" attraction and (3.) induced dipole attractions among/between nonpolar molecules.
We explained the relatively high melting points of ionic compounds which are due to the relatively strong attractive forces among ionically bonded cations and anions. We then began to account for the LACK of electrical conductivity of salts in the solid phase but the good electrical conductivity of molten/liquid salts.
Bonding practice test worksheets will be posted this weekend. Start work on them for next Thursday's Bonding Unit multiple choice exam. There are 200 points left to be earned this quarter.
Regents: We will go over the Bonding test on Monday; whatever you got wrong, you will have to write corrections (make SURE that they are perfect or you will lose points from your test) for homework.
We began the second half of the bonding unit in which we make the DISTINCTION between a true BOND and a mere intermolecular "attraction". There are three and ONLY three true types of BONDS: (1.) ionic (2.) covalent (3.) metallic .
A MUCH weaker form of attraction exists in certain cases: (1.) ion-dipole attractions (as in aqueous solutions of ions) (2.) dipole-dipole attractions among/between polar molecules; there is an extreme form of this attraction UNFORTUNATELY named "hydrogen bonding" attraction and (3.) induced dipole attractions among/between nonpolar molecules.
AP: we discussed catalysts and how they increase the number of effective collisions per second (by lowering the activation energy for an effective collision via orienting the reactant particle(s) properly) which increases the rate of a reaction.
We then discussed and graphically illustrated average and instantaneous rates of reaction, the rate units ( M per second).
We also related the rate of disappearance of a reactant to the rate of appearance of a product and related BOTH of those to the measured rate of reaction. To do this, we use the coefficients from the balanced equation.
Kinetics HW is posted on the website.
Honors: we began the second half of the bonding unit in which we make the DISTINCTION between a true BOND and a mere intermolecular "attraction". There are three and ONLY three true types of BONDS: (1.) ionic (2.) covalent (3.) metallic .
A MUCH weaker form of attraction exists in certain cases: (1.) ion-dipole attractions (as in aqueous solutions of ions) (2.) dipole-dipole attractions among/between polar molecules; there is an extreme form of this attraction UNFORTUNATELY named "hydrogen bonding" attraction and (3.) induced dipole attractions among/between nonpolar molecules.
We explained the relatively high melting points of ionic compounds which are due to the relatively strong attractive forces among ionically bonded cations and anions. We then began to account for the LACK of electrical conductivity of salts in the solid phase but the good electrical conductivity of molten/liquid salts.
Bonding practice test worksheets will be posted this weekend. Start work on them for next Thursday's Bonding Unit multiple choice exam. There are 200 points left to be earned this quarter.
Regents: We will go over the Bonding test on Monday; whatever you got wrong, you will have to write corrections (make SURE that they are perfect or you will lose points from your test) for homework.
We began the second half of the bonding unit in which we make the DISTINCTION between a true BOND and a mere intermolecular "attraction". There are three and ONLY three true types of BONDS: (1.) ionic (2.) covalent (3.) metallic .
A MUCH weaker form of attraction exists in certain cases: (1.) ion-dipole attractions (as in aqueous solutions of ions) (2.) dipole-dipole attractions among/between polar molecules; there is an extreme form of this attraction UNFORTUNATELY named "hydrogen bonding" attraction and (3.) induced dipole attractions among/between nonpolar molecules.
AP: we discussed catalysts and how they increase the number of effective collisions per second (by lowering the activation energy for an effective collision via orienting the reactant particle(s) properly) which increases the rate of a reaction.
We then discussed and graphically illustrated average and instantaneous rates of reaction, the rate units ( M per second).
We also related the rate of disappearance of a reactant to the rate of appearance of a product and related BOTH of those to the measured rate of reaction. To do this, we use the coefficients from the balanced equation.
Kinetics HW is posted on the website.
Thursday, January 11, 2007
Thursday, Day 1
AP: we put Bonding to bed by reviewing various intermolecular and interparticle attractions and related them to various physical properties of substances.
We began Kinetics (Chapter 14 in the text) by discussing the factors that determine or influence the rate of a chemical reaction by affecting collision frequency (Temperature, Concentration, Pressure (for gases), Surface Area (for liquids and solids)) or collision force (Temperature). As Joe noted after class, the big FIFTH factor is the presence of a CATALYST which affects neither of the above two factors yet, by providing a different chemical mechanism/pathway that LOWERS the activation energy required for an EFFECTIVE collision, the EFFECTIVE collision frequency increases.
Notes to be posted later...
KINETICS NOTES are posted on edline.com for now...go there.
Honors: had our Bonding II exam; I haven't looked at them yet other than a glimpse at an ATROCITY: a couple of you COVALENTLY bonded a metal and a nonmetal!!! This is AFTER mandatory CORRECTIONS of that specific and unbelievable error from the last test. If you are just going through the motions and not learning anything from these corrections, you are guilty of wanton ignorance and your results will not improve.
There are two more tests for this quarter, next Thursday and the following Monday; that's it for the first half of the year.
Regents: had our Bonding II exam and our electrolyte lab, which we will be explaining this and next week.
Class average will be posted later...
RESULTS are in: class average was weak: 77 . Most who did poorly IGNORED my advice about DRAWING THINGS OUT and then looking for a matching answer. Learn from your mistakes or you will continue to make the same ones. The class WILL BE WRITING OUT full corrections on this test for hw.
We began Kinetics (Chapter 14 in the text) by discussing the factors that determine or influence the rate of a chemical reaction by affecting collision frequency (Temperature, Concentration, Pressure (for gases), Surface Area (for liquids and solids)) or collision force (Temperature). As Joe noted after class, the big FIFTH factor is the presence of a CATALYST which affects neither of the above two factors yet, by providing a different chemical mechanism/pathway that LOWERS the activation energy required for an EFFECTIVE collision, the EFFECTIVE collision frequency increases.
Notes to be posted later...
KINETICS NOTES are posted on edline.com for now...go there.
Honors: had our Bonding II exam; I haven't looked at them yet other than a glimpse at an ATROCITY: a couple of you COVALENTLY bonded a metal and a nonmetal!!! This is AFTER mandatory CORRECTIONS of that specific and unbelievable error from the last test. If you are just going through the motions and not learning anything from these corrections, you are guilty of wanton ignorance and your results will not improve.
There are two more tests for this quarter, next Thursday and the following Monday; that's it for the first half of the year.
Regents: had our Bonding II exam and our electrolyte lab, which we will be explaining this and next week.
Class average will be posted later...
RESULTS are in: class average was weak: 77 . Most who did poorly IGNORED my advice about DRAWING THINGS OUT and then looking for a matching answer. Learn from your mistakes or you will continue to make the same ones. The class WILL BE WRITING OUT full corrections on this test for hw.
Wednesday, January 10, 2007
Wednesday, Day 7
Honors: we covered multiple examples, with explanation, of substances that had polar bonds that were either polar molecules or nonpolar molecules. We accounted for molecular polarity in terms of 1. bond polarity 2. electronic geometry around the central atom which leads to 3. molecular geometry. These three factors determine how and why there is either a symmetric distribution of charge (nonpolar molecules) or an asymmetric distribution of charge (polar molecules with a definite single partial negative "side" and a definite partial positive "side"). We saw that, ANY time there is a lone pair or pairs of nonbonded electrons on the central atom, the molecule would ultimately be polar with the net partial negative "side" by the lone pair or pairs of electrons.
Make sure that you draw what you are trying to explain. REFER to your drawing with words or arrows or both! Good luck tomorrow!
Regents: we covered multiple examples, with explanation, of substances that had polar bonds that were either polar molecules or nonpolar molecules. We accounted for molecular polarity in terms of 1. bond polarity 2. electronic geometry around the central atom which leads to 3. molecular geometry. These three factors determine how and why there is a symmetric distribution of charge (nonpolar molecules) or an asymmetric distribution of charge (polar molecules with a definite single partial negative "side" and a definite partial positive "side"). We saw that, ANY time there is a lone pair or pairs of nonbonded electrons on the central atom, the molecule would ultimately be polar with the net partial negative "side" by the lone pair or pairs of electrons.
The class seems to be doing well with this topic. Study hard and write everything out on scrap before you choose your answers tomorrow. Good luck!
AP: We almost completed the all-important bonding unit by discussing network solids, molecular solids, ionic solids, ion-dipole attractions and their effect on solubility. We will wrap up the few remaining points on that topic and commence Kinetics tomorrow.
Make sure that you draw what you are trying to explain. REFER to your drawing with words or arrows or both! Good luck tomorrow!
Regents: we covered multiple examples, with explanation, of substances that had polar bonds that were either polar molecules or nonpolar molecules. We accounted for molecular polarity in terms of 1. bond polarity 2. electronic geometry around the central atom which leads to 3. molecular geometry. These three factors determine how and why there is a symmetric distribution of charge (nonpolar molecules) or an asymmetric distribution of charge (polar molecules with a definite single partial negative "side" and a definite partial positive "side"). We saw that, ANY time there is a lone pair or pairs of nonbonded electrons on the central atom, the molecule would ultimately be polar with the net partial negative "side" by the lone pair or pairs of electrons.
The class seems to be doing well with this topic. Study hard and write everything out on scrap before you choose your answers tomorrow. Good luck!
AP: We almost completed the all-important bonding unit by discussing network solids, molecular solids, ionic solids, ion-dipole attractions and their effect on solubility. We will wrap up the few remaining points on that topic and commence Kinetics tomorrow.
Tuesday, January 09, 2007
Tuesday, Day 6
AP: look for descriptive chem practice files on the website later. Commence practice for quiz later this week.
Honors: we covered the remaining required molecular geometry types and then began to relate BOND POLARITY and MOLECULAR GEOMETRY to MOLECULAR POLARITY. A simple rule to heed for any molecule that we will see in this course (it works for ALMOST all molecules in general): In your Lewis structure of a molecule, IF there are one or more lone (NONBONDED) pairs of electrons on the central atom, the molecule will guaranteed be polar because there will definitely be a partially negatively charged region/side by the nonbonded/lone pair(s) of electrons. Tomorrow, we will relate the polarity of the molecules to the TYPE of intermolecular attractions that are experienced among and between molecules.
Thursday, we will have a test on some of the material from the last test and all topics since that test (see notes and homework).
Regents: we covered the remaining required molecular geometry types and then began to relate BOND POLARITY and MOLECULAR GEOMETRY to MOLECULAR POLARITY. A simple rule to heed for any molecule that we will see in this course (it works for ALMOST all molecules in general): In your Lewis structure of a molecule, IF there are one or more lone (NONBONDED) pairs of electrons on the central atom, the molecule will guaranteed be polar because there will definitely be a partially negatively charged region/side by the nonbonded/lone pair(s) of electrons.
On Thursday, we will have our second bonding exam. There will be many questions on naming salts, molecules, acids, bases, and hydrates. The test will cover polar and nonpolar covalent bonds, ionic bonds, metallic bonds, molecular geometry, and a little bit of molecular polarity (or lack thereof).
Study your notes and do ALL of the homework problems/worksheets that have been posted!
Honors: we covered the remaining required molecular geometry types and then began to relate BOND POLARITY and MOLECULAR GEOMETRY to MOLECULAR POLARITY. A simple rule to heed for any molecule that we will see in this course (it works for ALMOST all molecules in general): In your Lewis structure of a molecule, IF there are one or more lone (NONBONDED) pairs of electrons on the central atom, the molecule will guaranteed be polar because there will definitely be a partially negatively charged region/side by the nonbonded/lone pair(s) of electrons. Tomorrow, we will relate the polarity of the molecules to the TYPE of intermolecular attractions that are experienced among and between molecules.
Thursday, we will have a test on some of the material from the last test and all topics since that test (see notes and homework).
Regents: we covered the remaining required molecular geometry types and then began to relate BOND POLARITY and MOLECULAR GEOMETRY to MOLECULAR POLARITY. A simple rule to heed for any molecule that we will see in this course (it works for ALMOST all molecules in general): In your Lewis structure of a molecule, IF there are one or more lone (NONBONDED) pairs of electrons on the central atom, the molecule will guaranteed be polar because there will definitely be a partially negatively charged region/side by the nonbonded/lone pair(s) of electrons.
On Thursday, we will have our second bonding exam. There will be many questions on naming salts, molecules, acids, bases, and hydrates. The test will cover polar and nonpolar covalent bonds, ionic bonds, metallic bonds, molecular geometry, and a little bit of molecular polarity (or lack thereof).
Study your notes and do ALL of the homework problems/worksheets that have been posted!
AP: Heed this!
AP: we had our Christmas vacation topics exam today. From a cursory look at your tests, only four (CLEARLY prepared) members of our class know what they are doing with these topics. The CARELESS errors due to LACK of proper annotation and use of units is going to result in SEVERE penalties on this and ALL future tests. Do NOT pretend that you do not know the requirements for a properly written exam. You could not claim naiveté even two months ago. Don't you realize by now that taking shortcuts and not explicitly labeling EVERYTHING in your answers always leads to confusion and wrong answers. Can't you be careful for your own sake? Merely memorizing formulas without THOROUGHLY and CLEARLY labeling what each variable means will not only NOT help you in AP Chemistry, but also you will lead you to illogical and insane answers. This was overtly apparent on some of the problems from your Thanksgiving assignment even though those were ALL review problems (on which you COULD HAVE asked for help!). Today, how is it that only two people could convert from percent mass to molarity???
If you had trouble with this test even though I went over any difficult questions in class (WHICH I DID NOT have to to do!) then you did not properly do the break assignment. When you have an assignment over a break, do NOT try to cram it in on the last few days of vacation. Do NOT try to learn a three week assignment in just one or two days before a test or by memorizing last year's test (that SHOULD be beneath you). That strategy, even if it "worked", will HURT you in the long run.
This course HAS NO VACATION (vacation starts on May 12th). There is NOT enough time in our schedule. You will have future "vacation" assignments. I post an extreme number of examples and tutorials for these assignments. You should do ALL of them or, at least enough of them so that, by the time you come back to school, you have great speed and facility with each problem type.
I CANNOT cover all of the major permutations of a given question type on EACH test (even though they are long tests!) so, in order to be prepared for the AP CHEM EXAM, you MUST do the preparation work/hw in this course! That is a MAJOR expectation of this course. That is the ONLY way to gain enough knowledge in a sufficient number of contexts in order to not be SURPRISED on the AP exam.
All of the questions on this test were asked in the most straightforward way. In the future, that may not be the case. Furthermore, only one of the two vacation topics was concentrated on this test. Our next test will have kinetics and some of the question types that we didn't get to on this test. We will have an in-class quiz later this week on descriptive chemistry and the transition metals chapter assignment.
If you had trouble with this test even though I went over any difficult questions in class (WHICH I DID NOT have to to do!) then you did not properly do the break assignment. When you have an assignment over a break, do NOT try to cram it in on the last few days of vacation. Do NOT try to learn a three week assignment in just one or two days before a test or by memorizing last year's test (that SHOULD be beneath you). That strategy, even if it "worked", will HURT you in the long run.
This course HAS NO VACATION (vacation starts on May 12th). There is NOT enough time in our schedule. You will have future "vacation" assignments. I post an extreme number of examples and tutorials for these assignments. You should do ALL of them or, at least enough of them so that, by the time you come back to school, you have great speed and facility with each problem type.
I CANNOT cover all of the major permutations of a given question type on EACH test (even though they are long tests!) so, in order to be prepared for the AP CHEM EXAM, you MUST do the preparation work/hw in this course! That is a MAJOR expectation of this course. That is the ONLY way to gain enough knowledge in a sufficient number of contexts in order to not be SURPRISED on the AP exam.
All of the questions on this test were asked in the most straightforward way. In the future, that may not be the case. Furthermore, only one of the two vacation topics was concentrated on this test. Our next test will have kinetics and some of the question types that we didn't get to on this test. We will have an in-class quiz later this week on descriptive chemistry and the transition metals chapter assignment.
Monday, January 08, 2007
Monday, Day 5
AP: we finished working the Clausius-Clayperon equation by showing it as the equation of a line of slope = - dHvap/R . We then derived the equation to the form that we used on Friday.
We then almost finished the Bonding Unit by reviewing ionic and covalent bonding; we finished with a discussion of metallic bonding as it relates to melting point, electrical conductivity, and malleability/ductility. We will finish the unit on Wednesday as we cover molecular solids, network solids and review induced dipole, dipole-dipole, and hydrogen "bonding" attractions.
Then, it's on to KINETICS! good times.
Honors: we finished our explanation of metallic bonding as it relates to melting point, electrical conductivity, and malleability/ductility. We then moved on to molecular geometry, which we derived from our Lewis dot structures of the molecules. The key is to focus on the number of electron regions around the central atom and to see whether there are nonbonding pairs of electrons on the central atom. Remember, EACH single, double, or triple bond counts as only ONE electron-REGION around the central atom!
Regents: we reviewed and practiced the five cases of molecular geometry: linear (2 or 3-atom molecules with NO central-atom lone pairs of electrons), bent or V-shaped (3-atom molecule with ONE or TWO lone pairs of electrons on the central atom), trigonal PLANAR (4-atom molecule with NO central-atom lone pairs of electrons) or trigonal PYRAMIDAL (4-atom molecule with ONE lone pair of electrons on the central atom) and TETRAHEDRAL (5-atom molecule with NO central-atom lone pairs of electrons).
We then almost finished the Bonding Unit by reviewing ionic and covalent bonding; we finished with a discussion of metallic bonding as it relates to melting point, electrical conductivity, and malleability/ductility. We will finish the unit on Wednesday as we cover molecular solids, network solids and review induced dipole, dipole-dipole, and hydrogen "bonding" attractions.
Then, it's on to KINETICS! good times.
Honors: we finished our explanation of metallic bonding as it relates to melting point, electrical conductivity, and malleability/ductility. We then moved on to molecular geometry, which we derived from our Lewis dot structures of the molecules. The key is to focus on the number of electron regions around the central atom and to see whether there are nonbonding pairs of electrons on the central atom. Remember, EACH single, double, or triple bond counts as only ONE electron-REGION around the central atom!
Regents: we reviewed and practiced the five cases of molecular geometry: linear (2 or 3-atom molecules with NO central-atom lone pairs of electrons), bent or V-shaped (3-atom molecule with ONE or TWO lone pairs of electrons on the central atom), trigonal PLANAR (4-atom molecule with NO central-atom lone pairs of electrons) or trigonal PYRAMIDAL (4-atom molecule with ONE lone pair of electrons on the central atom) and TETRAHEDRAL (5-atom molecule with NO central-atom lone pairs of electrons).
Saturday, January 06, 2007
Tuesday's AP Chem test
We've spent the past three days going over some of the more important or difficult topics from your Christmas assignment. Certainly then, questions regarding those topics will be on Tuesday's exam. The rest of the questions will be aligned with the topics from the assignment "objectives" file.
However, since we have not had a class discussion on metallic bonding and on network solids, I will leave questions regarding them for the next test, which will be mostly on Kinetics. Also, since I will hand back your transition metal assignments just on Monday, there won't be any questions regarding that assignment on this test but, there may be a quiz on that assignment, along with some descriptive chem questions (time to get heavily into that, now that you know of most reaction types) late next week.
So, quantitative and qualitative Raoult's Law, colligative properties, van 't Hoff factor, multiple unit conversion, phase diagram, Clausius-Clayperon (ack! CAREFUL with the UNITS!) etc. questions can and will be on this test. If you did the assignment in earnest, you will merely be repeating the question types with different numbers and substances, so, practice...practice.
However, since we have not had a class discussion on metallic bonding and on network solids, I will leave questions regarding them for the next test, which will be mostly on Kinetics. Also, since I will hand back your transition metal assignments just on Monday, there won't be any questions regarding that assignment on this test but, there may be a quiz on that assignment, along with some descriptive chem questions (time to get heavily into that, now that you know of most reaction types) late next week.
So, quantitative and qualitative Raoult's Law, colligative properties, van 't Hoff factor, multiple unit conversion, phase diagram, Clausius-Clayperon (ack! CAREFUL with the UNITS!) etc. questions can and will be on this test. If you did the assignment in earnest, you will merely be repeating the question types with different numbers and substances, so, practice...practice.
Bonding Review Soliloquy
I wrote most of this explanation last year so I want to pass it on to this year's classes, which are also overly enamored with the octet rule:
Though most of you can express the connection between attraction and potential energy or repulsion and potential energy, others have not expressed that connection and, instead, have developed a completely disproportionate sense of the relevance and/or importance of the octet "rule". This "rule" has about as much contribution/relevance to potential energy lowering or stabilization as an eyelash contributes to the mass of an ELEPHANT!
Before I reiterate what I said in class about "Attraction, Repulsion and Potential Energy", let me reiterate why the octet "rule" exists. Take a fluorine atom, for example. A fluorine atom tends to bond to ONE and ONLY ONE other fluorine atom to form a diatomic fluorine molecule (draw the Lewis structure for fluorine). Why is there no stable triatomic fluorine molecule? Once a fluorine atom shares its one unpaired electron with another fluorine atom, each F atom has eight valence electrons. ..very nice but no big deal. There is actually greater NET repulsion (which is ALWAYS energy raising) among electrons when the eighth electron enters the valence shell BUT the bond forms due to the competing and MORE SIGNIFICANT factor of the SIMULTANEOUS attraction of the TWO fluorine nuclei (Zeff = +7) for the shared PAIR of valence electrons. ALSO, this extra repulsion among electrons is compensated for A LITTLE BIT due to the (on average) symmetric distribution of the electrons about each nucleus when the s and p sublevels are completely filled (due to the symmetric orientation of s and p orbitals). This symmetric distribution of electrons causes a bit LESS repulsion compared to the amount of repulsion when there is an asymmetric electron distribution (i.e. when at atom has 6 or 7 valence electrons). If a third F atom bonded to the molecule, the central F atom would have 9 valence electrons! There is no "room" (due to too much electron-electron repulsion...i.e. Pauli Exclusion Principle, Aufbau Principle) for a 9th electron in the 2nd principal energy level (2s2 2p6) so that next bonded electron would have to go to the much higher potential energy 3rd principal energy level. This would have a destabilizing effect (higher potential energy = destabilizing by definition!) that outweighs the attraction that would occur from a third covalent bond. So, that is why most (BUT NOT even ALL!) non-metal atoms stop bonding once they acquire an octet.
To really blow your mind, let me tell you what you actually already know: a sodium atom, BY ITSELF, is MORE STABLE than a sodium ION (BY ITSELF in the gas phase, NOT in a lattice of cations and anions) even though the sodium ION has an octet in its outermost occupied PEL (principal energy level). Didn't think that you knew that? I bet that you do. Here's why: Recall the definition of ionization energy. Look at the table of ionization energies. Locate sodium. Aha, so energy is actually REQUIRED to remove the valence electron from sodium? YES! Why? Simple: the positive sodium nucleus attracts (with a Zeff of +1) the negative valence electron! Energy is required to overcome that attraction. Why doesn't sodium lose two electrons then? The octet rule? NOPE! The second ionization energy of Na is so high because, to remove the second electron, the attraction from the nucleus on that electron is +9 AND that electrons is in a closer (to the nucleus) principal energy level (which, by Coulomb’s Law means that there is greater electrostatic attraction); therefore, too much energy is required for Na to lose a second electron. See this by drawing a Bohr model of a sodium atom and a sodium +1 ion and calculating the Zeff's on each shell of electrons.
You object: Compared to sodium atoms, why is the sodium +1 ion so stable and unreactive then? ahaaa! Sodium ions are NEVER by themselves! They are ionically BONDED (VERY STABILIZING!!!) to any and all surrounding anions in the salt’s crystal lattice! (draw lattice of ions in a typical salt)...or, as you will learn, in any aqueous solution or mixture (saliva, blood, the ocean), cations are surrounded by the partially negatively charged oxygen end of a water molecule (very stabilizing).
Now, the main reason for potential energy lowering WHENEVER a bond is formed: (write this out and MEMORIZE it if you have to)
COVALENT BONDS: the (negative) electrons that are SHARED between the (positive) nuclei will ALWAYS have a potential energy LOWERING effect (whether OR NOT there is an octet of electrons i.e. in Hydrogen) because:
1. positive particles (protons) attract negative particles (electrons) and vice versa
2. attraction is BY DEFINITION potential energy LOWERING!
3. relative to having a valence electron around one nucleus (an atom), there is MORE ATTRACTION when a valence electron is shared/located BETWEEN TWO NUCLEI (a COVALENT BOND BETWEEN two atoms’ nuclei) because there is more positive charge (protons from TWO nuclei) surrounding negative charge (shared valence electrons) and vice versa!
IONIC BONDS: again, the octet rule is practically IRRELEVANT to ionic bonding:
the actual potential energy lowering effect is due almost SOLELY to:
1. here, the CATIONS are the positive particles and the ANIONS are the negative particles in all ionic bonds.
2. positive particles attract negative particles and vice versa
3. attraction is BY DEFINITION potential energy LOWERING!
4. since electrons are NOT shared in ionic bonds, mentioning them as a significant final factor is meaningless. Each cation can have (depending on the geometric arrangement of ions in the lattice) four, six, or maybe even eight ionic BONDS to surrounding anions and vice versa! Each INDIVIDUAL ionic bond is between TWO fully charged IONS of opposite charge. The fact that the cations and the anions each have (usually) an octet of outermost PEL electrons is a MINOR almost irrelevant factor.
That is the relationship among Attraction, Repulsion, and Potential Energy. Pass it on.
Though most of you can express the connection between attraction and potential energy or repulsion and potential energy, others have not expressed that connection and, instead, have developed a completely disproportionate sense of the relevance and/or importance of the octet "rule". This "rule" has about as much contribution/relevance to potential energy lowering or stabilization as an eyelash contributes to the mass of an ELEPHANT!
Before I reiterate what I said in class about "Attraction, Repulsion and Potential Energy", let me reiterate why the octet "rule" exists. Take a fluorine atom, for example. A fluorine atom tends to bond to ONE and ONLY ONE other fluorine atom to form a diatomic fluorine molecule (draw the Lewis structure for fluorine). Why is there no stable triatomic fluorine molecule? Once a fluorine atom shares its one unpaired electron with another fluorine atom, each F atom has eight valence electrons. ..very nice but no big deal. There is actually greater NET repulsion (which is ALWAYS energy raising) among electrons when the eighth electron enters the valence shell BUT the bond forms due to the competing and MORE SIGNIFICANT factor of the SIMULTANEOUS attraction of the TWO fluorine nuclei (Zeff = +7) for the shared PAIR of valence electrons. ALSO, this extra repulsion among electrons is compensated for A LITTLE BIT due to the (on average) symmetric distribution of the electrons about each nucleus when the s and p sublevels are completely filled (due to the symmetric orientation of s and p orbitals). This symmetric distribution of electrons causes a bit LESS repulsion compared to the amount of repulsion when there is an asymmetric electron distribution (i.e. when at atom has 6 or 7 valence electrons). If a third F atom bonded to the molecule, the central F atom would have 9 valence electrons! There is no "room" (due to too much electron-electron repulsion...i.e. Pauli Exclusion Principle, Aufbau Principle) for a 9th electron in the 2nd principal energy level (2s2 2p6) so that next bonded electron would have to go to the much higher potential energy 3rd principal energy level. This would have a destabilizing effect (higher potential energy = destabilizing by definition!) that outweighs the attraction that would occur from a third covalent bond. So, that is why most (BUT NOT even ALL!) non-metal atoms stop bonding once they acquire an octet.
To really blow your mind, let me tell you what you actually already know: a sodium atom, BY ITSELF, is MORE STABLE than a sodium ION (BY ITSELF in the gas phase, NOT in a lattice of cations and anions) even though the sodium ION has an octet in its outermost occupied PEL (principal energy level). Didn't think that you knew that? I bet that you do. Here's why: Recall the definition of ionization energy. Look at the table of ionization energies. Locate sodium. Aha, so energy is actually REQUIRED to remove the valence electron from sodium? YES! Why? Simple: the positive sodium nucleus attracts (with a Zeff of +1) the negative valence electron! Energy is required to overcome that attraction. Why doesn't sodium lose two electrons then? The octet rule? NOPE! The second ionization energy of Na is so high because, to remove the second electron, the attraction from the nucleus on that electron is +9 AND that electrons is in a closer (to the nucleus) principal energy level (which, by Coulomb’s Law means that there is greater electrostatic attraction); therefore, too much energy is required for Na to lose a second electron. See this by drawing a Bohr model of a sodium atom and a sodium +1 ion and calculating the Zeff's on each shell of electrons.
You object: Compared to sodium atoms, why is the sodium +1 ion so stable and unreactive then? ahaaa! Sodium ions are NEVER by themselves! They are ionically BONDED (VERY STABILIZING!!!) to any and all surrounding anions in the salt’s crystal lattice! (draw lattice of ions in a typical salt)...or, as you will learn, in any aqueous solution or mixture (saliva, blood, the ocean), cations are surrounded by the partially negatively charged oxygen end of a water molecule (very stabilizing).
Now, the main reason for potential energy lowering WHENEVER a bond is formed: (write this out and MEMORIZE it if you have to)
COVALENT BONDS: the (negative) electrons that are SHARED between the (positive) nuclei will ALWAYS have a potential energy LOWERING effect (whether OR NOT there is an octet of electrons i.e. in Hydrogen) because:
1. positive particles (protons) attract negative particles (electrons) and vice versa
2. attraction is BY DEFINITION potential energy LOWERING!
3. relative to having a valence electron around one nucleus (an atom), there is MORE ATTRACTION when a valence electron is shared/located BETWEEN TWO NUCLEI (a COVALENT BOND BETWEEN two atoms’ nuclei) because there is more positive charge (protons from TWO nuclei) surrounding negative charge (shared valence electrons) and vice versa!
IONIC BONDS: again, the octet rule is practically IRRELEVANT to ionic bonding:
the actual potential energy lowering effect is due almost SOLELY to:
1. here, the CATIONS are the positive particles and the ANIONS are the negative particles in all ionic bonds.
2. positive particles attract negative particles and vice versa
3. attraction is BY DEFINITION potential energy LOWERING!
4. since electrons are NOT shared in ionic bonds, mentioning them as a significant final factor is meaningless. Each cation can have (depending on the geometric arrangement of ions in the lattice) four, six, or maybe even eight ionic BONDS to surrounding anions and vice versa! Each INDIVIDUAL ionic bond is between TWO fully charged IONS of opposite charge. The fact that the cations and the anions each have (usually) an octet of outermost PEL electrons is a MINOR almost irrelevant factor.
That is the relationship among Attraction, Repulsion, and Potential Energy. Pass it on.
Friday, January 05, 2007
Friday, Day 4
Regents: today we learned how to name hydroxide bases, which are ionic compounds that consist of cations (usually metal cations) ionically bonded to hydroxide ions. We then began our lesson on molecular geometries while reviewing how to draw Lewis dot structures. Stick strictly to the rules and you will be able to draw the Lewis structure for any molecule. From these diagrams we will be able to tell the molecular geometry of a given molecule.
AP: we finished our discussion of ideal vs. "deviant" solutions with respect to Raoult's Law. We then looked at phase diagrams and discussed the triple point, critical point, and various phase equilibrium lines. We finished with an example of the powerful Clausius-Clayperon equation, with which we can project the vapor pressure of a given substance or possibly calculate the enthalpy of vaporization of a substance. Later, I will post the possible question types for Tuesday's exam.
Honors: we drew a picture of a typical metal in order to show the metallic bonding throughout the lattice of atoms. We related the picture to the explanation of how metallic bonding accounts for the 1. relatively high melting points, 2. relatively good electrical conductivity, and 3. malleability/ductility of metals.
Write out and rehearse the explanations until you UNDERSTAND each factor intuitively.
From today, here is the picture of the lattice of metal atoms that exhibit malleability; you have to click on the picture in order to see the animation:
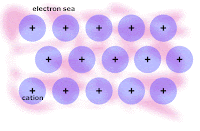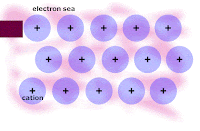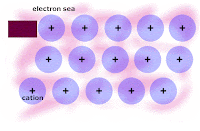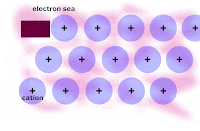
By contrast, here is a lattice of ions in a salt or a base. Salts and bases are not malleable, they are brittle thus they will crumble when hit with a hammer; click on the picture in order to see the animation:

AP: we finished our discussion of ideal vs. "deviant" solutions with respect to Raoult's Law. We then looked at phase diagrams and discussed the triple point, critical point, and various phase equilibrium lines. We finished with an example of the powerful Clausius-Clayperon equation, with which we can project the vapor pressure of a given substance or possibly calculate the enthalpy of vaporization of a substance. Later, I will post the possible question types for Tuesday's exam.
Honors: we drew a picture of a typical metal in order to show the metallic bonding throughout the lattice of atoms. We related the picture to the explanation of how metallic bonding accounts for the 1. relatively high melting points, 2. relatively good electrical conductivity, and 3. malleability/ductility of metals.
Write out and rehearse the explanations until you UNDERSTAND each factor intuitively.
From today, here is the picture of the lattice of metal atoms that exhibit malleability; you have to click on the picture in order to see the animation:

By contrast, here is a lattice of ions in a salt or a base. Salts and bases are not malleable, they are brittle thus they will crumble when hit with a hammer; click on the picture in order to see the animation:

Thursday, January 04, 2007
Thursday, Day 3
AP: we looked at Raoult's Law diagrams that show the composition of the liquid mixture and vapor mixture vs. Temperature. The stomata-shaped region between the two lines are equilibrium combinations of vapor and liquid phases of the mixture, though that region is not used in any calculations from these graphs. On the graph, we then showed the change in composition of the liquid and vapor mixtures per cycle of vaporization and condensation. These cycles occur repeatedly in any distillation apparatus. The simple distillation apparatus that is show in the text is good for just ONE cycle of vaporization and condensation. In real petroleum refineries (as you saw on last year's Regents exam), there are dozens of vaporization and distillation cycles per column so that methane, ethane, propane, octane, vaseline, tar, etc. can be separated efficiently.
We then went through three of the four cases that show ideal or deviant solution behavior (with respect to Raoult's Law).
For case one, a nearly ideal solution forms when the components of the mixture are as attracted to each other as they are to themselves, that is, A<->B = A<-->A = B<-->B. For that case, the delta H of solution is about 0 because there is not net gain or loss in intermolecular attraction as the components are mixed together.
We will review the other cases briefly tomorrow.
Regents: We learned how to name binary and ternary acids; using the worksheets and the links from the class website, practice naming the acids. We will cover naming of bases tomorrow and then we will get into part II of this unit: intermolecular attractions.
Honors: we finished learning how to name acids and then we covered hydroxide bases. Check the website for links and worksheets. We began to describe the nature of metallic bonding, which we will continue tomorrow.
We then went through three of the four cases that show ideal or deviant solution behavior (with respect to Raoult's Law).
For case one, a nearly ideal solution forms when the components of the mixture are as attracted to each other as they are to themselves, that is, A<->B = A<-->A = B<-->B. For that case, the delta H of solution is about 0 because there is not net gain or loss in intermolecular attraction as the components are mixed together.
We will review the other cases briefly tomorrow.
Regents: We learned how to name binary and ternary acids; using the worksheets and the links from the class website, practice naming the acids. We will cover naming of bases tomorrow and then we will get into part II of this unit: intermolecular attractions.
Honors: we finished learning how to name acids and then we covered hydroxide bases. Check the website for links and worksheets. We began to describe the nature of metallic bonding, which we will continue tomorrow.
Wednesday, January 03, 2007
three-day week
Welcome back to school! I hope that your New Year's resolutions included getting an A in Chemistry.
Honors: by Friday, MAKE SURE that you have your Bonding test fully, completely, and NEATLY corrected down to the most minute detail (including DRAWINGS, Zeff calculations, and electron transfers), especially for the explanation of the bonding process/potential energy diagram.
We reviewed the Stock naming system for molecules (NON-metal compounds!) and the common names for several molecules; then, we learned how to name binary and ternary acids. Don't forget, ALL binary acids begin with the TWO syllable prefix "HYDRO". NO ternary acids begin with "hydro" ; ternary acids change their polyatomic ion roots from "ate" to "ic" or from "ite" to "ous".
Check out the compound naming tutorial on the links to the left.
Tomorrow, we will continue with the naming of acids and bases.
Regents: we reviewed the Stock naming system for molecules
(NON-metal compounds!) and the common names for several molecules such as ammonia, methane, benzene,and glucose.
Check out the compound naming tutorial on the links to the left.
AP: we did a heteronuclear MO problem before segueing into Hess's Law for heats of solution. Whether the dissolving process for a given solute-solvent combination is exothermic or endothermic depends on the energy required to overcome the intermolecular or interparticle attractions among the solute particles in order to separate them and also the energy required to overcome the intermolecular or interparticle attractions among the solvent particles in order to separate them. The final factor is called the enthalpy of hydration or the enthalpy of solvation, which is a measure of the strength of attraction of solute to solvent particles/molecules.
We started to review Raoult's Law diagrams for an ideal solution (which obeys Raoult's Law). These diagrams will allow us to quantitatively figure the composition of the liquid and vapor phases during a distillation in which vaporization and condensation cycles repeatedly occur.
Honors: by Friday, MAKE SURE that you have your Bonding test fully, completely, and NEATLY corrected down to the most minute detail (including DRAWINGS, Zeff calculations, and electron transfers), especially for the explanation of the bonding process/potential energy diagram.
We reviewed the Stock naming system for molecules (NON-metal compounds!) and the common names for several molecules; then, we learned how to name binary and ternary acids. Don't forget, ALL binary acids begin with the TWO syllable prefix "HYDRO". NO ternary acids begin with "hydro" ; ternary acids change their polyatomic ion roots from "ate" to "ic" or from "ite" to "ous".
Check out the compound naming tutorial on the links to the left.
Tomorrow, we will continue with the naming of acids and bases.
Regents: we reviewed the Stock naming system for molecules
(NON-metal compounds!) and the common names for several molecules such as ammonia, methane, benzene,and glucose.
Check out the compound naming tutorial on the links to the left.
AP: we did a heteronuclear MO problem before segueing into Hess's Law for heats of solution. Whether the dissolving process for a given solute-solvent combination is exothermic or endothermic depends on the energy required to overcome the intermolecular or interparticle attractions among the solute particles in order to separate them and also the energy required to overcome the intermolecular or interparticle attractions among the solvent particles in order to separate them. The final factor is called the enthalpy of hydration or the enthalpy of solvation, which is a measure of the strength of attraction of solute to solvent particles/molecules.
We started to review Raoult's Law diagrams for an ideal solution (which obeys Raoult's Law). These diagrams will allow us to quantitatively figure the composition of the liquid and vapor phases during a distillation in which vaporization and condensation cycles repeatedly occur.

