Sunday, January 21, 2007
MISinformation
Though I generally do not find on the internet such horrifyingly bad information regarding simple scientific definitions, I did google some terms that were asked by your classmates and found the information to be misleading and dumb.
Here are two definitions that were given in class but that I will reiterate for you now:
COHESION vs. ADHESION:
Cohesion means attraction between and among the molecules of ONE SINGLE SUBSTANCE e.g. water molecules attracting water molecules via EXTREME dipole-dipole attractions/hydrogen-bonding-ATTRACTIONS.
Here is an image of water droplets "beading up" on a less polar substance's surface. The water molecules attract each other more than they are attracted to the molecules that make up the surface, thus, the water molecules cling together and form droplets:

Adhesion means attraction between and among the molecules/particles of TWO DIFFERENT SUBSTANCES e.g. water adheres to glass.
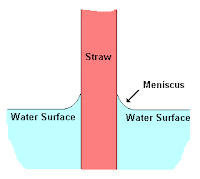
Here is an image and a diagram showing the adhesion of water molecules to the surface of a plastic straw; the plastic is made of some generally nonpolar polymer molecules:

Here are two definitions that were given in class but that I will reiterate for you now:
COHESION vs. ADHESION:
Cohesion means attraction between and among the molecules of ONE SINGLE SUBSTANCE e.g. water molecules attracting water molecules via EXTREME dipole-dipole attractions/hydrogen-bonding-ATTRACTIONS.
Here is an image of water droplets "beading up" on a less polar substance's surface. The water molecules attract each other more than they are attracted to the molecules that make up the surface, thus, the water molecules cling together and form droplets:

Adhesion means attraction between and among the molecules/particles of TWO DIFFERENT SUBSTANCES e.g. water adheres to glass.
Here is an image and a diagram showing the adhesion of water molecules to the surface of a plastic straw; the plastic is made of some generally nonpolar polymer molecules:

