Friday, January 05, 2007
Friday, Day 4
Regents: today we learned how to name hydroxide bases, which are ionic compounds that consist of cations (usually metal cations) ionically bonded to hydroxide ions. We then began our lesson on molecular geometries while reviewing how to draw Lewis dot structures. Stick strictly to the rules and you will be able to draw the Lewis structure for any molecule. From these diagrams we will be able to tell the molecular geometry of a given molecule.
AP: we finished our discussion of ideal vs. "deviant" solutions with respect to Raoult's Law. We then looked at phase diagrams and discussed the triple point, critical point, and various phase equilibrium lines. We finished with an example of the powerful Clausius-Clayperon equation, with which we can project the vapor pressure of a given substance or possibly calculate the enthalpy of vaporization of a substance. Later, I will post the possible question types for Tuesday's exam.
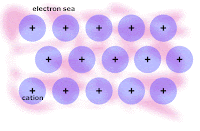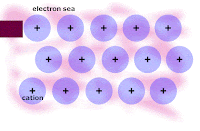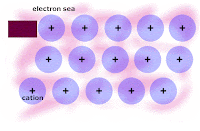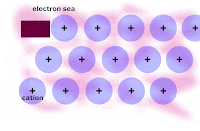
Honors: we drew a picture of a typical metal in order to show the metallic bonding throughout the lattice of atoms. We related the picture to the explanation of how metallic bonding accounts for the 1. relatively high melting points, 2. relatively good electrical conductivity, and 3. malleability/ductility of metals.
Write out and rehearse the explanations until you UNDERSTAND each factor intuitively.
From today, here is the picture of the lattice of metal atoms that exhibit malleability; you have to click on the picture in order to see the animation:
By contrast, here is a lattice of ions in a salt or a base. Salts and bases are not malleable, they are brittle thus they will crumble when hit with a hammer; click on the picture in order to see the animation:

AP: we finished our discussion of ideal vs. "deviant" solutions with respect to Raoult's Law. We then looked at phase diagrams and discussed the triple point, critical point, and various phase equilibrium lines. We finished with an example of the powerful Clausius-Clayperon equation, with which we can project the vapor pressure of a given substance or possibly calculate the enthalpy of vaporization of a substance. Later, I will post the possible question types for Tuesday's exam.
Honors: we drew a picture of a typical metal in order to show the metallic bonding throughout the lattice of atoms. We related the picture to the explanation of how metallic bonding accounts for the 1. relatively high melting points, 2. relatively good electrical conductivity, and 3. malleability/ductility of metals.
Write out and rehearse the explanations until you UNDERSTAND each factor intuitively.
From today, here is the picture of the lattice of metal atoms that exhibit malleability; you have to click on the picture in order to see the animation:

By contrast, here is a lattice of ions in a salt or a base. Salts and bases are not malleable, they are brittle thus they will crumble when hit with a hammer; click on the picture in order to see the animation:
