Tuesday, February 28, 2006
Testssss
AP: we have our first acid-base equilibrium exam tomorrow thus there is no AP exam hw question tonight (cheers!). I THINK that the class has a pretty good grip on the material except when you all were deer in headlights on the day back from vacation (jeers!). Do well on this exam and you will be well grounded for the more extensive acid-base part deux exam (cheers!). Also, tonight, if you must use every brain cell to study for the test, you can postpone handing in the butane lab until Thursday (cheers!).
Honors: test on Thursday on everything since Molarity. Make sure that you are more than bulletproof on the material from the past two practice quizzes (jeers?) as well as material since the last practice quiz. We will have approx. two more test questions on Friday (20 minutes test-time max. on Friday) to rack up a 150 point total (cheers!). Make sure that you are up to date on all hw worksheets, review book and text questions. Math of Chem mastery is all about repetition, repetition, repetition (jeers!).
Honors: test on Thursday on everything since Molarity. Make sure that you are more than bulletproof on the material from the past two practice quizzes (jeers?) as well as material since the last practice quiz. We will have approx. two more test questions on Friday (20 minutes test-time max. on Friday) to rack up a 150 point total (cheers!). Make sure that you are up to date on all hw worksheets, review book and text questions. Math of Chem mastery is all about repetition, repetition, repetition (jeers!).
Sunday, February 26, 2006
AP Q4 tip from a master
Take this tip from Prof. Adrian Dingle, the grandmaster of all AP Chem teachers:
Subject: Re: symbols used in chemical equations
One more note on balancing equations and question #4 on the exam. Here's a useful tip if you don't appreciate it already.
I teach my kids that non-metal oxide + water ==> acid. They have difficulty remembering which acid is produced. For example, when SO2 is bubbled into water is it H2SO3 or H2SO4? This question is answered by writing a BALANCED equation. I tell them to do that on scratch paper, and record the unbalanced equation on the answer sheet. Simple.
So, regarding his example, you would balance
SO2 + H2O --> H2SO3
which works out as is to a one to one to one ratio; sulfurous acid is weak and should be written as an un-ionized molecule.
For P4O10 (tetraphosphorus decoxide) + H2O --> H3PO4 or H3PO3 ?
with Phosphoric acid, the equation "works" and balances to
P4O10 + 6 H2O --> 4 H3PO4
whereas with H3PO3, the numbers do not balance...
More tips forthcoming as test day approaches.
Subject: Re: symbols used in chemical equations
One more note on balancing equations and question #4 on the exam. Here's a useful tip if you don't appreciate it already.
I teach my kids that non-metal oxide + water ==> acid. They have difficulty remembering which acid is produced. For example, when SO2 is bubbled into water is it H2SO3 or H2SO4? This question is answered by writing a BALANCED equation. I tell them to do that on scratch paper, and record the unbalanced equation on the answer sheet. Simple.
So, regarding his example, you would balance
SO2 + H2O --> H2SO3
which works out as is to a one to one to one ratio; sulfurous acid is weak and should be written as an un-ionized molecule.
For P4O10 (tetraphosphorus decoxide) + H2O --> H3PO4 or H3PO3 ?
with Phosphoric acid, the equation "works" and balances to
P4O10 + 6 H2O --> 4 H3PO4
whereas with H3PO3, the numbers do not balance...
More tips forthcoming as test day approaches.
Wednesday, February 22, 2006
AP Info
I posted some extra help files related to our Acid/Base unit as well as some O.C. files. We return on day 2 (I think) so we will have our first Acid/Base exam two days later (day 4, as usual). I will correct your winter assignment (if there are any corrections- there should not be any because this is an open book assignment and the rules of organic chemistry are completely unambiguous and explicit) that week so that you can get some feedback before you are tested on that material.
Speaking of Organic Chemistry related to our in-class discussion of pharmacology, I pose this question : Heroin crosses the "brain-blood barrier" 100 times faster than morphine because heroin is a lipid (fat)-soluble molecule whereas morphine is not as soluble in lipids. Look at the molecules and try to account for the difference in this physical property (solubility) of these physiologically similar (and horrifyingly dangerous- did you see "House" this week?) compounds. If you know the reason, mention it in class (for a gold star and nomination for class factotem!).
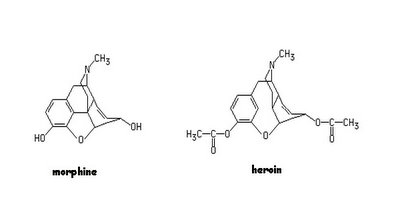
Speaking of Organic Chemistry related to our in-class discussion of pharmacology, I pose this question : Heroin crosses the "brain-blood barrier" 100 times faster than morphine because heroin is a lipid (fat)-soluble molecule whereas morphine is not as soluble in lipids. Look at the molecules and try to account for the difference in this physical property (solubility) of these physiologically similar (and horrifyingly dangerous- did you see "House" this week?) compounds. If you know the reason, mention it in class (for a gold star and nomination for class factotem!).

Friday, February 17, 2006
AP Winter Break Assignment

I posted a massive number of supplemental files for Organic Chemistry. Don't be overwhelmed. Just use what you need. The tutorials are nice; the supplemental notes can be useful and the practice tests have many questions and answers.
Part II of the assignment is forthcoming and will involve mostly answering some text questions.
I also posted our long-awaited Butane Lab assignment which is due March 1. If you somehow lost or do not have your data, email me and I can send you the data. There is also the related "Molar Volume of Hydrogen" writeup; use the data supplied for that lab. They key is that you know the lab procedures and that you can ace the "critical thinking" questions- you will see questions like those on "Question 5" of the AP Chem exam.
Monday, February 13, 2006
AP Stats
Finished grading the first of our ultra-important series of equilibrium exams. The chasm within the class continues and widens due to, among other factors, a significant disparity in the amount of preparation for exams and, even more importantly (!), the ability to follow direction/answer the question that is asked/ basic test-taking skills/ careLESSness. For example, when you write a number SO SMALL that even YOU can't read it or use it consistently, you are throwing points away! Another example, when you say that a substance increases in concentration and then go on to explain how that same substance DECREASES in concentration, you are throwing points out the window. Another, when you indiscriminately use numbers without units or with the wrong units (a crucial bookkeeping skill in this unit) you cannot get the correct answer. When you randomly abbreviate terms without a key, as if you are writing in a CHATROOM as opposed to taking an IMPORTANT EXAM, you are throwing points away. When you write an answer, correct value or not, but you provide no clue as to how you arrived at the answer (especially when this and ALL tests and the ACTUAL AP exam only give points for work/reasoning shown), you are throwing points away. If losing countless points due to careless errors does not change your behavior, I do not know what will. I can only state that almost nothing has changed. Do NOT repeat these careless errors. Critically read your exam before you hand it in. The aforementioned factors are within the ken and control of ANY student and should always be exercised.
5 Female students score range: 130 to 150 (mostly in the 140's)...kudos! keep up the great work!
5 Male students score range: 91 to 114.
Says it all.
No such disparity has occurred in any of my previous classes at ANY level.
One basic Regents-level question that MOST of you missed:
A (solid) --> B (gas) + C (gas)
when total pressure on the system at equilibrium increases, the concentration of B will...
Le Chatelier's Principle tells us that the system will shift towards making more reactant (A) to relieve the added pressure stress by decreasing the number of moles of gaseous substances ( B and C, which CAUSE the pressure in the container/system) in the system. Fewer moles of gas in the system leads to a decrease in pressure because solids do not contribute (practically at all) to the pressure of the system and fewer moles of gas causes a lower collision frequency between the gas particles and the container walls (thus lowering the pressure). THUS, the concentration of B will DECREASE (assuming constant volume conditions) as the reaction proceeds towards equilibrium.
Think of it this way, for simplicity: when you put enough pressure on any gas, it will solidify.
5 Female students score range: 130 to 150 (mostly in the 140's)...kudos! keep up the great work!
5 Male students score range: 91 to 114.
Says it all.
No such disparity has occurred in any of my previous classes at ANY level.
One basic Regents-level question that MOST of you missed:
A (solid) --> B (gas) + C (gas)
when total pressure on the system at equilibrium increases, the concentration of B will...
Le Chatelier's Principle tells us that the system will shift towards making more reactant (A) to relieve the added pressure stress by decreasing the number of moles of gaseous substances ( B and C, which CAUSE the pressure in the container/system) in the system. Fewer moles of gas in the system leads to a decrease in pressure because solids do not contribute (practically at all) to the pressure of the system and fewer moles of gas causes a lower collision frequency between the gas particles and the container walls (thus lowering the pressure). THUS, the concentration of B will DECREASE (assuming constant volume conditions) as the reaction proceeds towards equilibrium.
Think of it this way, for simplicity: when you put enough pressure on any gas, it will solidify.
AP Exam Prep
This week, starting with Monday's homework, we commence AP Chem exam prep. Each night, one or more actual AP exam questions will be assigned and then collected in class the next day. There are a mere 86 days till the AP Chem exam so I want you all to have lots of experience with actual AP exam questions. You will learn how the questions are worded and you will also get to practice question types that you may not have seen in a while.
Make sure that you complete these homeworks daily and hand them in at the BEGINNING of class the next day. I will post the answers after I return the hw to you; check your answers carefully. If you have trouble with a question, see me at extra help or email me before the hw is due.
p.s. just found a nice interactive, instant-feedback site for some quick rate law practice (you can never get too much of that) here: http://chemistry2.csudh.edu/homework/hwkinetics.html
Make sure that you complete these homeworks daily and hand them in at the BEGINNING of class the next day. I will post the answers after I return the hw to you; check your answers carefully. If you have trouble with a question, see me at extra help or email me before the hw is due.
p.s. just found a nice interactive, instant-feedback site for some quick rate law practice (you can never get too much of that) here: http://chemistry2.csudh.edu/homework/hwkinetics.html
Sunday, February 12, 2006
Dihydrogen Monoxide, OH MY!

As you probably know by now, school is closed on Monday. Once again, the dangerous chemical, "dihydrogen monoxide", in its perilous, solid form, has been found on most Long Island roads. Though most of this odious compound has been plowed away and mixed with mollifying amounts of sodium chloride, authorities consider it imprudent to venture outside with the remnants of this noxious chemical still present on our roads.
Naturally, this will strain our already terribly strained schedule; we will have to compensate and make due. Check the website tomorrow for some hw that may help us stay apace of our schedule.
Good night and good sledding.
Mr.C.
Guess who's back....winter's back....
Depending upon how much salt (or sugar! delta tizzle equals kizzle izzle mizzle) is laid down on the roads tonight, we have a four-day or fewer work-week. Check the SAHS home page for school-closing messages: http://www.stanthonyshs.org/
Honors will have a Math of Chem quiz or test on Wednesday. The winter break assignment will be a comprehensive take-home review test (100 questions-you must show how you solved each question). You do have nine days to complete the test.
AP- Acid-Base quiz this week. The winter break assignment involves mastering the organic chem topic and also reviewing both metal-ligand complexes and descriptive chem. Those of you who never forgot organic naming and drawing isomers will have a much easier time completing the assignment. For those just learning how to name organic compounds without the crutch of a reference table, you have a LOT of practice ahead of you. You will be tested on those topics upon your return from break. (In the past, those who did not learn the material in the assignment got a 50 or lower on this test so don't just look up the answers, LEARN the rules and names).
Stay warm,
Mr. Cicale
Honors will have a Math of Chem quiz or test on Wednesday. The winter break assignment will be a comprehensive take-home review test (100 questions-you must show how you solved each question). You do have nine days to complete the test.
AP- Acid-Base quiz this week. The winter break assignment involves mastering the organic chem topic and also reviewing both metal-ligand complexes and descriptive chem. Those of you who never forgot organic naming and drawing isomers will have a much easier time completing the assignment. For those just learning how to name organic compounds without the crutch of a reference table, you have a LOT of practice ahead of you. You will be tested on those topics upon your return from break. (In the past, those who did not learn the material in the assignment got a 50 or lower on this test so don't just look up the answers, LEARN the rules and names).
Stay warm,
Mr. Cicale
Wednesday, February 08, 2006
Le Chat chat
AP: okay, here's a transcript of what I wanted to say today. I think that this will be a good Le Chatelier review of the temperature and pressure stresses:
inc P (or dec V) is the same as increasing the concentrations of any gases, so there is an increase in collision frequency and therefore a greater forward and reverse reaction rate (due to the greater number of molecules available for collision per unit of volume = inc concentration); an inc in conc of all gases on the side with MORE molecules causes a disproportionately greater inc in that side's reaction rate so there is a NET shift toward the side with FEWER molecules as the new equilibrium is reached.
dec P (or inc V) is the same as a decrease in concentration of all gases so there is a decrease in collision frequency and therefore a decreased forward and reverse reaction rate (due to the lower number of molecules available for collision per unit of volume = dec concentration); so, a dec in conc of all molecules causes a disproportionately greater decrease in reaction rate on the side with more molecules; thus, there is a NET shift towards the side with more molecules as the new equilibrium is reached.
inc T speeds up both forward and reverse reaction rates because there is increased collision frequency and a greater fraction of effective collisions (due to the higher T = higher avg. KE of the molecules in the system) but there will be a disproportionately larger increase in the NET "energy consuming", endothermic, direction.
dec T slows down forward and reverse reaction rates because there is decreased collision frequency and a decreased fraction of effective collisions due to the lower T = lower avg. KE of the molecules in the system) but there is disproportionately greater decrease in the endothermic direction so that there is a net shift to the EXOTHERMIC direction.
Of course, giving specific examples with made up numbers for the forward and reverse rates is the BEST thing that you can do because you can then quantitatively show towards which side (reactants or products) a NET shift occurs as the system proceeds towards the new equilibrium.
more stresses...
addition of a catalyst: a catalyst affects the orientation of the colliding reactant(s) by temporatily binding the reactant(s) in such a way that bonds are strained (and thus require less energy to break) or interparticle attractions are weakened (so the less energy is needed to overcome the attractions); thus, a catalyst lowers the activation energy for both the forward and reverse reaction. Catalysts lower the activation energy of both forward and reverse reactions EQUALLY. Therefore, though both the forward and reverse reaction rates increase (because, at the same temperature, a greater fraction of reactant particles have enough kinetic energy for an effective collision due to the activation energy- lowering effect of the catalyst), there is NO NET shift towards the reactants or products because both rates are increased EQUALLY.
addition of an INERT gas:
addition of any non-reacting substance will NOT affect the equilibrium concentrations of the gases (or anything) as long as the system is at CONSTANT VOLUME!!! This is because, at constant volume, even if you add many many moles of an inert gas, the PARTIAL PRESSURES and, thus, the CONCENTRATIONS of the reactant and product gases remain CONSTANT because the number of MOLES per LITER of the reactant gases is NOT CHANGING as the inert gas is added.
BUT, if an inert gas is added at constant TOTAL pressure i.e. the VOLUME expands, the effect is to LOWER the PARTIAL PRESSURES/concentrations of the reactant and product gases (initially). So, just treat that situation as an INCREASED VOLUME or DECREASED PRESSURE stress. Thus, the equilibrium will shift to the side with a greater number of moles of gaseous molecules (in the balanced equation) due to the decrease in BOTH the forward and reverse reaction rates but a disproportionately greater decrease in the reaction involving the side with a greater number of moles of gaseous molecules.
inc P (or dec V) is the same as increasing the concentrations of any gases, so there is an increase in collision frequency and therefore a greater forward and reverse reaction rate (due to the greater number of molecules available for collision per unit of volume = inc concentration); an inc in conc of all gases on the side with MORE molecules causes a disproportionately greater inc in that side's reaction rate so there is a NET shift toward the side with FEWER molecules as the new equilibrium is reached.
dec P (or inc V) is the same as a decrease in concentration of all gases so there is a decrease in collision frequency and therefore a decreased forward and reverse reaction rate (due to the lower number of molecules available for collision per unit of volume = dec concentration); so, a dec in conc of all molecules causes a disproportionately greater decrease in reaction rate on the side with more molecules; thus, there is a NET shift towards the side with more molecules as the new equilibrium is reached.
inc T speeds up both forward and reverse reaction rates because there is increased collision frequency and a greater fraction of effective collisions (due to the higher T = higher avg. KE of the molecules in the system) but there will be a disproportionately larger increase in the NET "energy consuming", endothermic, direction.
dec T slows down forward and reverse reaction rates because there is decreased collision frequency and a decreased fraction of effective collisions due to the lower T = lower avg. KE of the molecules in the system) but there is disproportionately greater decrease in the endothermic direction so that there is a net shift to the EXOTHERMIC direction.
Of course, giving specific examples with made up numbers for the forward and reverse rates is the BEST thing that you can do because you can then quantitatively show towards which side (reactants or products) a NET shift occurs as the system proceeds towards the new equilibrium.
more stresses...
addition of a catalyst: a catalyst affects the orientation of the colliding reactant(s) by temporatily binding the reactant(s) in such a way that bonds are strained (and thus require less energy to break) or interparticle attractions are weakened (so the less energy is needed to overcome the attractions); thus, a catalyst lowers the activation energy for both the forward and reverse reaction. Catalysts lower the activation energy of both forward and reverse reactions EQUALLY. Therefore, though both the forward and reverse reaction rates increase (because, at the same temperature, a greater fraction of reactant particles have enough kinetic energy for an effective collision due to the activation energy- lowering effect of the catalyst), there is NO NET shift towards the reactants or products because both rates are increased EQUALLY.
addition of an INERT gas:
addition of any non-reacting substance will NOT affect the equilibrium concentrations of the gases (or anything) as long as the system is at CONSTANT VOLUME!!! This is because, at constant volume, even if you add many many moles of an inert gas, the PARTIAL PRESSURES and, thus, the CONCENTRATIONS of the reactant and product gases remain CONSTANT because the number of MOLES per LITER of the reactant gases is NOT CHANGING as the inert gas is added.
BUT, if an inert gas is added at constant TOTAL pressure i.e. the VOLUME expands, the effect is to LOWER the PARTIAL PRESSURES/concentrations of the reactant and product gases (initially). So, just treat that situation as an INCREASED VOLUME or DECREASED PRESSURE stress. Thus, the equilibrium will shift to the side with a greater number of moles of gaseous molecules (in the balanced equation) due to the decrease in BOTH the forward and reverse reaction rates but a disproportionately greater decrease in the reaction involving the side with a greater number of moles of gaseous molecules.
Tuesday, February 07, 2006
Tests
Honors: Study up for this Wednesday's Stoichiometry I exam.
AP: Thursday, we will have the first of a series of tests on chemical equilibria- the single most important topic in AP Chem. You should do literally dozens of each type of equilibrium problem; there is far too great a variety of these problems for us to cover each permutation in class. Also, there will be a descriptive chem set on this test.
Our next "5 Steps to a 5" review session will be on Thursday @ 3PM in Room 235.
AP: Thursday, we will have the first of a series of tests on chemical equilibria- the single most important topic in AP Chem. You should do literally dozens of each type of equilibrium problem; there is far too great a variety of these problems for us to cover each permutation in class. Also, there will be a descriptive chem set on this test.
Our next "5 Steps to a 5" review session will be on Thursday @ 3PM in Room 235.
Saturday, February 04, 2006
Calculator Solver and Graphing Help
Specifically for this unit , you may want to make extensive use of the "solver" function in your Ti-83 calculator. (If you have a Ti-89, just talk to it and it will obey your every command; you can even control other people's mind with it- see page 857 of the Ti-89 instruction manual).
Here are a few sites that may help you:
http://www.acad.sunytccc.edu/instruct/sbrown/ti83/quadrat.htm#Enter
http://www.ncsu.edu/felder-public/kenny/papers/ti.html#SOLVER
This link shows how to graph the best fit line and to diagnose how well the data fits a straight line (r^2 = 1.000). We used this info to plot the Arrhenius and Clausius-Clayperon equations and we will use it for the nearly identical van t' Hoff equation. (The equations are almost identical because all three equations are based on/ caused by the distribution of molecular speeds/KE's at a given temperature!).
http://www.keypress.com/SIA/downloads/CalculatorGuidePDF/SIA_Calc_Guide_Ch03.pdf
For Ti-89:
solve any equation: http://pages.infinit.net/carl/TI89tutoen.html#equation
http://www.prenhall.com/divisions/esm/app/calc_v2/calculator/medialib/Technology/Documents/TI-89/desc_pages/ti89techskills2.html
Here are a few sites that may help you:
http://www.acad.sunytccc.edu/instruct/sbrown/ti83/quadrat.htm#Enter
http://www.ncsu.edu/felder-public/kenny/papers/ti.html#SOLVER
This link shows how to graph the best fit line and to diagnose how well the data fits a straight line (r^2 = 1.000). We used this info to plot the Arrhenius and Clausius-Clayperon equations and we will use it for the nearly identical van t' Hoff equation. (The equations are almost identical because all three equations are based on/ caused by the distribution of molecular speeds/KE's at a given temperature!).
http://www.keypress.com/SIA/downloads/CalculatorGuidePDF/SIA_Calc_Guide_Ch03.pdf
For Ti-89:
solve any equation: http://pages.infinit.net/carl/TI89tutoen.html#equation
http://www.prenhall.com/divisions/esm/app/calc_v2/calculator/medialib/Technology/Documents/TI-89/desc_pages/ti89techskills2.html
AP MC Practice
I have linked to this site before so this is just a reminder:
http://www.adriandingleschemistrypages.com/apquiz.html
The site has very brief (5 questions per set) multiple-choice quizzes sorted by topic.
The questions were developed by Prof. Adrian Dingle, who has a yearly AP average of about 4.8 even though he teaches only SOPHOMORES who are taking first year Chem (granted, he teaches at a ritzy private academy in GA where everyone sips tea and talks like Stewie).
Most of the quizzes can be completed and scored in two to three minutes! So, practice with these whenever you have some free time or when you are waiting for IM replies.
Did you sign into that descriptive chem site yet? You should do so because there are more DC quizzes coming your way...now, back to your ICE tables!
http://www.adriandingleschemistrypages.com/apquiz.html
The site has very brief (5 questions per set) multiple-choice quizzes sorted by topic.
The questions were developed by Prof. Adrian Dingle, who has a yearly AP average of about 4.8 even though he teaches only SOPHOMORES who are taking first year Chem (granted, he teaches at a ritzy private academy in GA where everyone sips tea and talks like Stewie).
Most of the quizzes can be completed and scored in two to three minutes! So, practice with these whenever you have some free time or when you are waiting for IM replies.
Did you sign into that descriptive chem site yet? You should do so because there are more DC quizzes coming your way...now, back to your ICE tables!
Wednesday, February 01, 2006
AP Kp answer
The answer to the Kp problem from class today is:
The partial pressure of oxygen at equilibrium is 347 atm. That is crazy high but consider that the reaction takes place at 1000 K and the Kp is extremely large!
Ask about this tomorrow if your answer is different.
The partial pressure of oxygen at equilibrium is 347 atm. That is crazy high but consider that the reaction takes place at 1000 K and the Kp is extremely large!
Ask about this tomorrow if your answer is different.