Thursday, November 30, 2006
Thursday
Honors: we finished up the remaining periodic trends in electron affinity (attraction) and electronegativity. All of these trends will ultimately be related to what elements react with what other elements and how and why they react together - that's our next unit: chemical bonding and attractions.
Don't forget the three main CAUSES of all periodic trends that all relate to Coulomb's Law, which rules over all of chem and physics: (1) Zeff= the MOST important factor in determining the attraction of a nucleus on its electrons (2) OPEL's, which indicate the distance that the electron is from the nucleus, and (3) electron-electron repulsion in a given PEL or between electrons in different sublevels of a PEL.
We have a multiple choice test tomorrow on all notes between and including electron configurations and periodic trends. Don't forget about isoelectronic species and how to compare them.
Regents: we went through the difference between ionic (metal cation to nonmetal anion) and covalent (a nonmetal atom sharing valence electrons with a nonmetal atom) bonds. We related this to salts (ionic) and molecules (covalent).
Tomorrow is one of THE most important lessons of the year: how to NAME compounds and how to DETERMINE the FORMULA of any simple compound based on which elements are combined.
AP: more Lewis structures and shapes; on resonance structures we applied the TOOL of "formal charge" to PREDICT which resonance structure contributes MOST SIGNIFICANTLY to the real structure of the compound. Formal charge is in NO WAY, SHAPE or FORM, actual electric or ionic charge! It is just a little game that chemists can play to predict reality. That game is still on the AP exam even though we have quantum mechanics so that we don't have to play games anymore. Most people don't have QM supercomputers, though, so we will play the "formal charge" game; that works most of the time.
We have "electron-deficient" molecules and "expanded octets" tomorrow. Good times.
Don't forget the three main CAUSES of all periodic trends that all relate to Coulomb's Law, which rules over all of chem and physics: (1) Zeff= the MOST important factor in determining the attraction of a nucleus on its electrons (2) OPEL's, which indicate the distance that the electron is from the nucleus, and (3) electron-electron repulsion in a given PEL or between electrons in different sublevels of a PEL.
We have a multiple choice test tomorrow on all notes between and including electron configurations and periodic trends. Don't forget about isoelectronic species and how to compare them.
Regents: we went through the difference between ionic (metal cation to nonmetal anion) and covalent (a nonmetal atom sharing valence electrons with a nonmetal atom) bonds. We related this to salts (ionic) and molecules (covalent).
Tomorrow is one of THE most important lessons of the year: how to NAME compounds and how to DETERMINE the FORMULA of any simple compound based on which elements are combined.
AP: more Lewis structures and shapes; on resonance structures we applied the TOOL of "formal charge" to PREDICT which resonance structure contributes MOST SIGNIFICANTLY to the real structure of the compound. Formal charge is in NO WAY, SHAPE or FORM, actual electric or ionic charge! It is just a little game that chemists can play to predict reality. That game is still on the AP exam even though we have quantum mechanics so that we don't have to play games anymore. Most people don't have QM supercomputers, though, so we will play the "formal charge" game; that works most of the time.
We have "electron-deficient" molecules and "expanded octets" tomorrow. Good times.
Wednesday, November 29, 2006
Wednesday Wrap
AP: we began the all-important method for writing correct Lewis structures. These structures are amazingly practical and accurate as tools for determining molecular geometry, polarity, and even reactivity! We will draw hundreds of these structures and soon you will be able to draw them quickly, clearly, and accurately (with proper geometry, too...with dots of Truth, Justice, and the American way!).
At parties, your popularity will soar!
Honors: we explained the trend in successive ionization energies and we predicted the disproportionate increase in ionization energy based on how many valence electrons an element has.
This led to Matt's PROFOUND observation of the reason why REPRESENTATIVE (non-transition) metal atoms lose ONLY their valence electrons in chemical reactions: non-valence electrons are NOT ONLY closer to the nucleus of a given atom, BUT ALSO non-valence electrons experience a MUCH higher Zeff. Therefore, it would be highly DEstabilizing to the ion to lose strongly attracted electrons; furthermore, removing non-valence electrons requires tremendous energy.
We then almost finished the trends in electron affinity and electronegativity across a period and down a group.
Regents: we took our SECOND periodic table exam...results soon...
then, in lab, we tested the reactivity of alkaline earth metals and Aluminum, a Group 13 metal.
Back to Bonding tomorrow and Friday...
OKAY, here are the results from today's exam: drum roll...class average (NOT including the TWO students who don't study or pay attention and who, if they do not DRASTICALLY, RADICALLY, AND COMPLETELY CHANGE their behavior and practices and take advantage of the ridiculous amounts of extra help available to them, are GUARANTEED going to fail the class and the Regents exam! Very frustrating...oh, back to the rest of the class: 86 ...not bad...not bad at all! A mere four points away (one to two more correct per student) from celebrating and crowing about your performance. I AM impressed with the number of students who are maintaining a 90-plus average and the number of students who have significantly increased their averages thus far this quarter. Keep up the good work!
At parties, your popularity will soar!
Honors: we explained the trend in successive ionization energies and we predicted the disproportionate increase in ionization energy based on how many valence electrons an element has.
This led to Matt's PROFOUND observation of the reason why REPRESENTATIVE (non-transition) metal atoms lose ONLY their valence electrons in chemical reactions: non-valence electrons are NOT ONLY closer to the nucleus of a given atom, BUT ALSO non-valence electrons experience a MUCH higher Zeff. Therefore, it would be highly DEstabilizing to the ion to lose strongly attracted electrons; furthermore, removing non-valence electrons requires tremendous energy.
We then almost finished the trends in electron affinity and electronegativity across a period and down a group.
Regents: we took our SECOND periodic table exam...results soon...
then, in lab, we tested the reactivity of alkaline earth metals and Aluminum, a Group 13 metal.
Back to Bonding tomorrow and Friday...
OKAY, here are the results from today's exam: drum roll...class average (NOT including the TWO students who don't study or pay attention and who, if they do not DRASTICALLY, RADICALLY, AND COMPLETELY CHANGE their behavior and practices and take advantage of the ridiculous amounts of extra help available to them, are GUARANTEED going to fail the class and the Regents exam! Very frustrating...oh, back to the rest of the class: 86 ...not bad...not bad at all! A mere four points away (one to two more correct per student) from celebrating and crowing about your performance. I AM impressed with the number of students who are maintaining a 90-plus average and the number of students who have significantly increased their averages thus far this quarter. Keep up the good work!
Tuesday, November 28, 2006
Tuesday recap
Honors: we explained the relative sizes of cations and anions to their respective atoms; then we explained the periodic trends in first ionization energy across a period and down a group; we also explained the unforeseen anomalies to this trend in terms of electron shielding and repulsion. We then began to explain the trend in successive ionization energies.
We just have to finish that and cover electronegativity and electron affinity trends and then we will know just about everything that causes "chemistry" to occur.
We will apply this knowledge in our next unit: chemical bonding and attractions.
Regents: we reviewed for the multiple-choice exam tomorrow. The exam covers the entire periodic table unit which necessarily requires knowledge of atomic structure and electron configurations.
Then, we discussed the two types of chemical bonds: the ionic bond (which you will see in salts) and the covalent bond (which you will see in molecules).
AP: put the periodicity unit to bed and launched right into our unit on bonding and attractions. This is the ultimate unit in explaining practical things in chemistry. In this unit, we also get to do molecular geometry and relate that to molecular polarity. Soon, you will see that you can figure out things in seconds that, in the beginning, required minutes.
Big fun, basically.
We just have to finish that and cover electronegativity and electron affinity trends and then we will know just about everything that causes "chemistry" to occur.
We will apply this knowledge in our next unit: chemical bonding and attractions.
Regents: we reviewed for the multiple-choice exam tomorrow. The exam covers the entire periodic table unit which necessarily requires knowledge of atomic structure and electron configurations.
Then, we discussed the two types of chemical bonds: the ionic bond (which you will see in salts) and the covalent bond (which you will see in molecules).
AP: put the periodicity unit to bed and launched right into our unit on bonding and attractions. This is the ultimate unit in explaining practical things in chemistry. In this unit, we also get to do molecular geometry and relate that to molecular polarity. Soon, you will see that you can figure out things in seconds that, in the beginning, required minutes.
Big fun, basically.
Monday, November 27, 2006
Back to Play
It has been a while; welcome back everyone! Thanks for staying awake on a low-sleep day.
AP: The test today was one of the longest of the year but, if you did well, you covered a lot of ground and set the table for our bonding unit. The empirical formula lab critical thinking responses were pretty weak, on average, so set aside some time to go over those questions at extra help. Of course, we could have WORKED THROUGH the assignment at extra help before you handed it in so that you would have received full credit. That is going to be well worth the time for many of you next time. More importantly, you will see these questions on your AP exam; specifically, questions that involve an experimental error and its influence on the calculated results. You need to become expert at those; that takes time and effort because these questions involve the highest level of thinking/analysis.
Regents: we tied some loose ends and did some review for WEDNESDAY's COMPREHENSIVE Periodic Table unit exam. In fact, I have a couple of more loose ends to add tomorrow so that every possible question type that can appear on the Regents has been covered.
We introduced the new and MOST IMPORTANT unit in all of chemistry: Chemical Bonding and Attractions. You will see that this unit is the CULMINATION (THAT is the word that I was looking for in class today!) of the past two units on atomic structure and the periodic table. Good times.
Your Thanksgiving Assignment is due tomorrow in class.
Honors: we thoroughly discussed the factors that cause the trends in atomic size across a period and down a group. We also compared and explained the relative sizes of atoms and the stable ions that typically form from the atoms as a result of chemical reactions. Tomorrow, we will cover more trends and, you will notice, that we use the same factors and, often, the same explanations for each of the trends.
AP: The test today was one of the longest of the year but, if you did well, you covered a lot of ground and set the table for our bonding unit. The empirical formula lab critical thinking responses were pretty weak, on average, so set aside some time to go over those questions at extra help. Of course, we could have WORKED THROUGH the assignment at extra help before you handed it in so that you would have received full credit. That is going to be well worth the time for many of you next time. More importantly, you will see these questions on your AP exam; specifically, questions that involve an experimental error and its influence on the calculated results. You need to become expert at those; that takes time and effort because these questions involve the highest level of thinking/analysis.
Regents: we tied some loose ends and did some review for WEDNESDAY's COMPREHENSIVE Periodic Table unit exam. In fact, I have a couple of more loose ends to add tomorrow so that every possible question type that can appear on the Regents has been covered.
We introduced the new and MOST IMPORTANT unit in all of chemistry: Chemical Bonding and Attractions. You will see that this unit is the CULMINATION (THAT is the word that I was looking for in class today!) of the past two units on atomic structure and the periodic table. Good times.
Your Thanksgiving Assignment is due tomorrow in class.
Honors: we thoroughly discussed the factors that cause the trends in atomic size across a period and down a group. We also compared and explained the relative sizes of atoms and the stable ions that typically form from the atoms as a result of chemical reactions. Tomorrow, we will cover more trends and, you will notice, that we use the same factors and, often, the same explanations for each of the trends.
Saturday, November 25, 2006
Regents Class Assignment Answers
Answers/Explanations are posted. For full credit, copy those answers neatly, clearly, and exactly (VERBATIM). Do NOT hand in anything that is typed. All answers must be in excellent handwriting. You will frequently use these charts when we are doing Regents review in May and June.
In the long and short term, it will be to your advantage to study these charts and explanations.
Regents Class: since it took me a while to get these answers posted, your assignment due date is changed to TUESDAY, instead of Monday.
Also, our class will have another test on the Periodic Table unit this Wednesday and this assignment is a very good partial preparation for that test.
In the long and short term, it will be to your advantage to study these charts and explanations.
Regents Class: since it took me a while to get these answers posted, your assignment due date is changed to TUESDAY, instead of Monday.
Also, our class will have another test on the Periodic Table unit this Wednesday and this assignment is a very good partial preparation for that test.
Friday, November 24, 2006
Honors Assignment
Part II of the assignment is posted. The test is 15 multiple-choice questions BUT, on separate paper, be sure to show your work or reasoning or calculation for EACH question. Do so even if the work shown is merely the formal definition of a term in the question and how your answer matches that definition but the others do not. If the correct work is not shown for a given question, no credit will be given for that question.
See you all on Monday.
See you all on Monday.
Thursday, November 23, 2006
AP T-day Assignment misprint
Oops, on question number 8, the electron numbers are misprints. I have now corrected them. The highest energy electrons in Se would be the 32nd, 33rd, and 34th electrons, of course.
Thanks to Joshua for the spot on that!
Mr. C.
Major Genetics Discovery: CNV
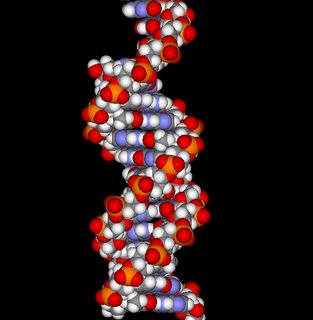
Just when you thought that every major facet of Biology had been discovered and catalogued, a significant new finding in Genetics emerges. Mapping the human genome, scuentists have found the significance of CNV: copy number variation; that is, each individual, perhaps even identical twins, can have a different number of copies of a given allele.
Here is a more user-friendly article on the research.
Wednesday, November 22, 2006
Honors Thanksgiving Assignment

Sorry for the delay in getting this posted: I immediately passed out after the interviews last night. Then, today, I helped out a friend in somewhat dire need; that involved driving to MiddleofNowhere, NJ on the heaviest traffic day of the year. Eight hours later, I am home so I can finally post the T-day assignment.
The assignment has two parts:
Part I- On looseleaf paper, for the first 36 elements on the periodic table, write the (1) name of the element, e.g. Aluminum, (2) the symbol of the element, e.g. Al, (3) the Bohr model electron configuration of the element ( e.g. 2-8-3) , (4) in order of increasing sublevel ENERGY, the quantum mechanical model electron configuration of the element (with s, p, d, etc...e.g. 1s2 2s2 2p6 3s2 3p1), (5) the accompanying orbital diagram (you know, the labeled SQUARE boxes, with up and down arrows for opposite spin electrons) of the element WITH THE VALENCE ELECTRONS in a DIFFERENT COLOR INK. Do NOT draw pictures of orbital shapes!!! Those SHAPES are NOT orbital diagrams! I warned and ranted about that before the last test to NO AVAIL. For electron configurations and for orbital diagrams, you SHOULD abbreviate the kernel/core electrons of the matching previous noble gas (from group 18) in brackets, e.g. [Ne] 3s2 3p1.
Your papers (the 36 elements will take you several pages) should have five columns (portrait or landscape format; landscape might be easier because it is wider than it is long), the first four columns should be much narrower than the last column because the orbital diagrams will take up the most space. Write NEATLY, CLEARLY, and accurately otherwise points will be deducted.
Part II- A short-answer review test covering some of the important objectives for the Regents or SATII. I will post that on our class website later tonight.
I hope that you have a Happy Thanksgiving filled with lots of food, fun, and family. Never take for granted how fortunate you are; for those less fortunate, you know what I am talking about.
Regents Class Thanksgiving Assignment
In order to reinforce the material from our last unit, the Thanksgiving assignment will involve drawing out and explaining periodic trends on periodic table "templates"= blank tables. I will post the tables on the website. I will EVEN print out the answers. Just try to do the assignment without looking at the answers first. Then, if you are incorrect, draw a new table and write out the correct answer. I should have the templates posted on the website later today.
Also, as you did for the Atomic Concepts unit, you are assigned to write out, neatly and fully, the COMPLETE definitions of the scientific terms found in the Orange Review Book- Topic 5: The Periodic Table page 63 in the "Vocabulary" section- twelve terms starting with "atomic radius". For yourself, you may want to give an example of each term also, if you can.
You will receive 50 points towards your second quarter average if your assignment is done clearly, legibly, and perfectly. Do NOT type your assignment; it must be handwritten.
Also, as you did for the Atomic Concepts unit, you are assigned to write out, neatly and fully, the COMPLETE definitions of the scientific terms found in the Orange Review Book- Topic 5: The Periodic Table page 63 in the "Vocabulary" section- twelve terms starting with "atomic radius". For yourself, you may want to give an example of each term also, if you can.
You will receive 50 points towards your second quarter average if your assignment is done clearly, legibly, and perfectly. Do NOT type your assignment; it must be handwritten.
Tuesday, November 21, 2006
Thanksgiving-Eve Eve
AP: we barely got through a few points about the periodic trend in basicity to acidity of oxides (the group 13 and 14 elements are more amphoteric). We did a couple of equation-writing examples and then finished up with properties and group trends of the alkali ( "ends with an "I" = Roman numeral ONE = Group 1) and alkaline earth ( TWO words = Group 2) metals.
The Thanksgiving assignment is online. Our test on day six will cover (1) full explanations of all periodic trends, including anomalies (data WILL be given for any anomaly) in terms of Zeff, OPEL's and electron-electron repulsion, (2) electron configurations and quantum numbers, especially the configurations of transition metal ions (careful! first in- first out rule!), (3) full explanation of exceptions to the Aufbau Principle (do not dare misspell or abuse principle vs. principal...you will be using both terms heavily!) in Cr, Cu, Ag, or Mo.
MAKE sure that your explanations HEAVILY rely upon illustrations and orbital diagrams i.e. draw what is going on and then REFER to the relevant parts of your drawing throughout your explanation. If you ignore this advice (as many do), you may find that your answers lack sufficient detail and clarity (as many do).
Regents: we finished up our Periodic Table Unit, which will be tested next week (likely on Wednesday). We begin the most important unit in chemistry: Bonding, on Monday after our break. The Bonding unit relies heavily on your prior knowledge from the Atomic Concepts and the Periodic Table units. So, if you had trouble with those units, you are setting yourself up for failure if you don't come to extra help and clarify ANYTHING and everything that you didn't understand from the two previous units. Look over past unit homeworks, worksheets, and notes and bring to extra help any questions that you didn't understand.
Honors: We began the ultimate in explanations of all elemental properties and periodic trends: the calculation and relation of Zeff on an electron to the electron's attraction to the nucleus. The second major factor that is the number of OCCUPIED (by electrons) princiPAL energy levels in an atom. The third and final factor that we will use to explain everything in chemistry is the amount/degree of electron-electron repulsion in a given princiPAL energy level.
That's what we will cover all next week.
The Thanksgiving assignment is online. Our test on day six will cover (1) full explanations of all periodic trends, including anomalies (data WILL be given for any anomaly) in terms of Zeff, OPEL's and electron-electron repulsion, (2) electron configurations and quantum numbers, especially the configurations of transition metal ions (careful! first in- first out rule!), (3) full explanation of exceptions to the Aufbau Principle (do not dare misspell or abuse principle vs. principal...you will be using both terms heavily!) in Cr, Cu, Ag, or Mo.
MAKE sure that your explanations HEAVILY rely upon illustrations and orbital diagrams i.e. draw what is going on and then REFER to the relevant parts of your drawing throughout your explanation. If you ignore this advice (as many do), you may find that your answers lack sufficient detail and clarity (as many do).
Regents: we finished up our Periodic Table Unit, which will be tested next week (likely on Wednesday). We begin the most important unit in chemistry: Bonding, on Monday after our break. The Bonding unit relies heavily on your prior knowledge from the Atomic Concepts and the Periodic Table units. So, if you had trouble with those units, you are setting yourself up for failure if you don't come to extra help and clarify ANYTHING and everything that you didn't understand from the two previous units. Look over past unit homeworks, worksheets, and notes and bring to extra help any questions that you didn't understand.
Honors: We began the ultimate in explanations of all elemental properties and periodic trends: the calculation and relation of Zeff on an electron to the electron's attraction to the nucleus. The second major factor that is the number of OCCUPIED (by electrons) princiPAL energy levels in an atom. The third and final factor that we will use to explain everything in chemistry is the amount/degree of electron-electron repulsion in a given princiPAL energy level.
That's what we will cover all next week.
Monday, November 20, 2006
Monday Monday
AP, Honors, and Regents Thanksgiving Vacation assignments and homework will be posted late tonight/tomorrow. The assignments (and for Regents and Honors, the homework also) will be graded and count towards your second quarter average. ALL WORK MUST BE EXPLICITLY, CLEARLY, and NEATLY SHOWN on these assignments (or you will not receive much credit). Assume that you are writing for someone who has NO knowledge of chemistry and physics. Make sure that your work is done and handed in during class on our return from vacation on Monday.
Honors: we traversed the Periodic Table and learned of the various group names and some physical/chemical properties of the groups/families of elements. We discussed properties of metals, nonmetals, and semi-metals/metalloids. We then introduced the Periodic Trends that we will completely explain over the next few classes. These lessons are the MOST IMPORTANT lessons of the ENTIRE year. Take excellent notes. Then, copy them again and again until they are COMPLETELY intuitive and internalized. If you know the material in next few lessons, then you will truly understand the HOW and WHY of chemistry.
Regents: We reviewed our last test, emphasizing the importance of DRAWING things out (Bohr Models of atoms or ions) and showing calculations (Zeff) and looking things up on the Reference Tables as a check on your knowledge of periodic trends. We then discussed individual groups of elements. We should be able to close out the unit tomorrow.
AP: we finished electron affinity and then we looked at physical and chemical properties down a group and across a period.
We also did some descriptive chem reactions of metal oxides in water or in acid and then nonmetal oxides in water or in base.
We may be able to close out the unit tomorrow. Either way, our exam on Day 6 will be on the material covered through tomorrow.
Honors: we traversed the Periodic Table and learned of the various group names and some physical/chemical properties of the groups/families of elements. We discussed properties of metals, nonmetals, and semi-metals/metalloids. We then introduced the Periodic Trends that we will completely explain over the next few classes. These lessons are the MOST IMPORTANT lessons of the ENTIRE year. Take excellent notes. Then, copy them again and again until they are COMPLETELY intuitive and internalized. If you know the material in next few lessons, then you will truly understand the HOW and WHY of chemistry.
Regents: We reviewed our last test, emphasizing the importance of DRAWING things out (Bohr Models of atoms or ions) and showing calculations (Zeff) and looking things up on the Reference Tables as a check on your knowledge of periodic trends. We then discussed individual groups of elements. We should be able to close out the unit tomorrow.
AP: we finished electron affinity and then we looked at physical and chemical properties down a group and across a period.
We also did some descriptive chem reactions of metal oxides in water or in acid and then nonmetal oxides in water or in base.
We may be able to close out the unit tomorrow. Either way, our exam on Day 6 will be on the material covered through tomorrow.
Saturday, November 18, 2006
Interesting Articles
One article is about the consequences of "Grade Inflation": a phenomenon that knows no bounds at almost all schools and all grade levels. Some local public school districts, in order to fraudulently inflate their AP rosters, are giving a 25% grade boost to the AP course grade of each AP student. Got a 70? Let's magically count that as an 88 . That will change your C (that's right, a 70 IS a C at almost all of the schools in this country) to a nice B+ (or an A at some schools). Good thing that sports do not have "point inflation" (though some leagues do) otherwise a scrub like Ben Zobrist would have a batting average as high as that of Derek Jeter. Sorry Ben..
Another article is about SAT proctoring. No test can be perfectly standardized due to the impossibility of controlling all variables that might affect the test-taking conditions but that doesn't excuse the almost wanton neglect that occurs at some SAT sites. I don't know the frequency of such occurrences but, if something untoward occurs during your SAT exam, NOTIFY the College Board.
Another article is about SAT proctoring. No test can be perfectly standardized due to the impossibility of controlling all variables that might affect the test-taking conditions but that doesn't excuse the almost wanton neglect that occurs at some SAT sites. I don't know the frequency of such occurrences but, if something untoward occurs during your SAT exam, NOTIFY the College Board.
Friday, November 17, 2006
AP Lab "Critical Thinking" Question 1
This came up at extra help on Friday: I should have written the first "Critical Thinking" question a bit differently. If you have already done that question, you don't have to change your answer but, if you haven't done "Critical Thinking" Question 1 yet, just change it to the following:
Assume that there were some water droplets on the evaporating dish; since the water droplets contribute to the mass of the evaporating dish, for THIS question, CHANGE the combined mass of the evaporating dish and Zinc to a value GREATER than 41.11 grams (because the water droplets contribute mass to the dish but assume that you used the SAME amount of Zinc as in the original data...remember, you didn't SEE the tiny water droplets in or on the dish BUT they will be vaporized away during the evaporation process). Then, do the rest of the calculation to see whether the empirical formula of zinc chloride obtained is the same as or different from what you got by using the correct lab procedure data.
Any questions, email me.
Sorry for the mix-up. How about this? You can hand the lab in on Tuesday! Better times.
Assume that there were some water droplets on the evaporating dish; since the water droplets contribute to the mass of the evaporating dish, for THIS question, CHANGE the combined mass of the evaporating dish and Zinc to a value GREATER than 41.11 grams (because the water droplets contribute mass to the dish but assume that you used the SAME amount of Zinc as in the original data...remember, you didn't SEE the tiny water droplets in or on the dish BUT they will be vaporized away during the evaporation process). Then, do the rest of the calculation to see whether the empirical formula of zinc chloride obtained is the same as or different from what you got by using the correct lab procedure data.
Any questions, email me.
Sorry for the mix-up. How about this? You can hand the lab in on Tuesday! Better times.
Auctioneer Friday
Another abbreviated class period today...get ready for yet another one on Tuesday...
AP: we finished the electronegativity trend emphasizing Zeff and the PEL of the shared electrons as the determining factors while considering electron-electron repulsion to be an insignificant factor for this property.
Then, we covered "electron affinity" and noted several anomalies (always draw the orbital diagram and the anomalies will be explicable). Remember, for ANY anomaly on ANY AP exam, the data WILL be given because anomalies are NOT predictable BUT they can be explained in terms of Zeff, OPELS, shielding, and electron-electron repulsion.
Regents: we finished our Periodic Table trends and explanations thereof in terms of Zeff and OPEL's. We will wrap up the unit by talking more about individual groups of elements and some other facts about elements on the Periodic Table. Do the Orange Review Book homework and the LAB writeup this weekend.
Honors: we talked about Mr. Moseley and his deduction of the nuclear charge of each element. He used x-ray emission data from each element and related the x-ray frequencies to what must be the positive nuclear charge. He and Rutherford then related the nuclear charge to the number of protons in the nucleus of an atom. The Periodic Table was then changed to an arrangement in order of increasing ATOMIC NUMBER. Thus, the Te/I anomaly went away and the elements displayed a more orderly periodic change across each period and the elements were chemically similar in each group. We began to discuss the three main types of elements on the Periodic Table: the metals, the semi-metals/metalloids, and the nonmetals. Do the text hw and the lab writeup over the weekend.
Thanks.
AP: we finished the electronegativity trend emphasizing Zeff and the PEL of the shared electrons as the determining factors while considering electron-electron repulsion to be an insignificant factor for this property.
Then, we covered "electron affinity" and noted several anomalies (always draw the orbital diagram and the anomalies will be explicable). Remember, for ANY anomaly on ANY AP exam, the data WILL be given because anomalies are NOT predictable BUT they can be explained in terms of Zeff, OPELS, shielding, and electron-electron repulsion.
Regents: we finished our Periodic Table trends and explanations thereof in terms of Zeff and OPEL's. We will wrap up the unit by talking more about individual groups of elements and some other facts about elements on the Periodic Table. Do the Orange Review Book homework and the LAB writeup this weekend.
Honors: we talked about Mr. Moseley and his deduction of the nuclear charge of each element. He used x-ray emission data from each element and related the x-ray frequencies to what must be the positive nuclear charge. He and Rutherford then related the nuclear charge to the number of protons in the nucleus of an atom. The Periodic Table was then changed to an arrangement in order of increasing ATOMIC NUMBER. Thus, the Te/I anomaly went away and the elements displayed a more orderly periodic change across each period and the elements were chemically similar in each group. We began to discuss the three main types of elements on the Periodic Table: the metals, the semi-metals/metalloids, and the nonmetals. Do the text hw and the lab writeup over the weekend.
Thanks.
Thursday, November 16, 2006
Thurs.
Test Day...
How did you do?
Regents class, your first test of the second quarter: average 83. There was one 100 and nobody scored below a 67. With only four choices per question, the class should have at least been able to hit an 85 average; that would have been the case if more of the class would come to extra help.
Some students improved significantly from previous tests though some dropped off. Those THREE OR FOUR students who came to EXTRA HELP WITH QUESTIONS FROM THEIR HOMEWORK did very well; that is not a coincidence. Join the club, people. There should be standing room only for two days before a test. Don't make excuses even if you can come to extra help for only 15 minutes.
Honors: tests will be graded perhaps by Thanksgiving...depends on how many students answered what was asked for. Get right back into the Periodic Table tomorrow.
AP: continued our explanations and accounting for anomalies in first ionization energy across a period. We then did successive IE's and we started electronegativity. More on that tomorrow, then electron affinity. By Monday or Tuesday, we should be done with the unit and onto the HEART of chemistry: Bonding and Attractions.
How did you do?
Regents class, your first test of the second quarter: average 83. There was one 100 and nobody scored below a 67. With only four choices per question, the class should have at least been able to hit an 85 average; that would have been the case if more of the class would come to extra help.
Some students improved significantly from previous tests though some dropped off. Those THREE OR FOUR students who came to EXTRA HELP WITH QUESTIONS FROM THEIR HOMEWORK did very well; that is not a coincidence. Join the club, people. There should be standing room only for two days before a test. Don't make excuses even if you can come to extra help for only 15 minutes.
Honors: tests will be graded perhaps by Thanksgiving...depends on how many students answered what was asked for. Get right back into the Periodic Table tomorrow.
AP: continued our explanations and accounting for anomalies in first ionization energy across a period. We then did successive IE's and we started electronegativity. More on that tomorrow, then electron affinity. By Monday or Tuesday, we should be done with the unit and onto the HEART of chemistry: Bonding and Attractions.
AGAIN
I hope that I will NEVER have to say this again: Whenever you take ANY test (especially in my class), make SURE, BEFORE you waste time writing an irrelevant answer, that you UNDERSTAND and EXPLICITLY follow the directions in the question. Take your rehearsed answers and throw them in the garbage IF they do not answer the question that is ACTUALLY asked on the exam! Within the first two minutes of each Honors test today, I saw students rushing and writing answers that were NOT even asked for on the exam. NOT ONLY will you NOT receive credit for information that is NOT ASKED for (OR, if you write something different from HOW you are supposed to write the answer, as directed by the question), BUT ALSO you may lose points if your unnecessary information is incorrect. A brief glance at the tests showed that about 2 in 5 students are STILL IGNORING THE QUESTIONS! Your underlining or circling keywords is a MEANINGLESS waste of time if you actively refuse to learn anything by not using that technique in any discriminating manner. Some are still indiscriminately underlining key terms long after their irrelevant answers have been given.
This is the second quarter. You must no longer ignore the MOST IMPORTANT test-taking skill that I have emphasized since DAY ONE of this course: to answer what is asked for in the question!
AP: an alarming number of you made UNSUPPORTED statements in the Planck/Einstein Photoelectric Effect question. If you claim that red light has lower energy than blue light after I clearly stated that the total energy of red light used was greater, you are ignoring and contradicting the information in the question. Furthermore, even if you said that red light has less energy PER PHOTON than blue light has, anyone can say that your claim is FALSE. It is your job to prove your claim in an explanation. You needed a MERE sentence to prove your claim. This mere sentence is the absolute pith/core/heart to understanding the whole experiment: according to Planck's Theory, the energy PER QUANTUM/PHOTON is proportional to the FREQUENCY of the photon's associated wave of light; since red light has a lower frequency than blue light (THIS IS EXPERIMENTALLY MEASURED AND NEED NOT BE EXPLAINED), red light must have a lower energy PER PHOTON (the most important ratio in the whole explanation without which the phenomenon CANNOT be explained), than blue light. Furthermore, many of you still do not realize that, in Planck's particle terminology, light intensity is proportional to the number of photons transmitted PER SECOND. Learn this. Know this.
This is the second quarter. You must no longer ignore the MOST IMPORTANT test-taking skill that I have emphasized since DAY ONE of this course: to answer what is asked for in the question!
AP: an alarming number of you made UNSUPPORTED statements in the Planck/Einstein Photoelectric Effect question. If you claim that red light has lower energy than blue light after I clearly stated that the total energy of red light used was greater, you are ignoring and contradicting the information in the question. Furthermore, even if you said that red light has less energy PER PHOTON than blue light has, anyone can say that your claim is FALSE. It is your job to prove your claim in an explanation. You needed a MERE sentence to prove your claim. This mere sentence is the absolute pith/core/heart to understanding the whole experiment: according to Planck's Theory, the energy PER QUANTUM/PHOTON is proportional to the FREQUENCY of the photon's associated wave of light; since red light has a lower frequency than blue light (THIS IS EXPERIMENTALLY MEASURED AND NEED NOT BE EXPLAINED), red light must have a lower energy PER PHOTON (the most important ratio in the whole explanation without which the phenomenon CANNOT be explained), than blue light. Furthermore, many of you still do not realize that, in Planck's particle terminology, light intensity is proportional to the number of photons transmitted PER SECOND. Learn this. Know this.
What's on the (Periodic) Table
pretty koolio links to interactive periodic tables with visualizations and info about your favorite elements:
all classes, check them out:
http://www.colorado.edu/physics/2000/applets/a2.html
This one has everything you always wanted to know but were afraid to ask:
http://www.webelements.com/
nice pics and diagrams...
http://www.lenntech.com/periodic-chart.htm
All classes, here is a link to a site that has videos on periodic trends:
click on the "Student Tutorials" link and enjoy the video explanations/descriptions.
http://cwx.prenhall.com/bookbind/pubbooks/petrucci8/chapter10/deluxe.html
AP: same site but this is the chapter on electron configurations and quantum numbers of the Schroedinger equation.
http://cwx.prenhall.com/bookbind/pubbooks/petrucci8/chapter9/deluxe.html
all classes, check them out:
http://www.colorado.edu/physics/2000/applets/a2.html
This one has everything you always wanted to know but were afraid to ask:
http://www.webelements.com/
nice pics and diagrams...
http://www.lenntech.com/periodic-chart.htm
All classes, here is a link to a site that has videos on periodic trends:
click on the "Student Tutorials" link and enjoy the video explanations/descriptions.
http://cwx.prenhall.com/bookbind/pubbooks/petrucci8/chapter10/deluxe.html
AP: same site but this is the chapter on electron configurations and quantum numbers of the Schroedinger equation.
http://cwx.prenhall.com/bookbind/pubbooks/petrucci8/chapter9/deluxe.html
Wednesday, November 15, 2006
Wednesday 11/15 Recap
AP: we explained the Periodic Trend in atomic size/radius across a Period (from left to right) and down a group in terms of Zeff, OPEL's, and electron-electron repusion. We didn't get into the minutiae of HOW scientists measure atomic size (there are various ways, each of which give consistent but different values).
We then explained atom to ion size differences for cations and anions. We explained size differences among isoelectronic particles.
We began to explain the trend in ionization energy across a Period; we will continue to do so tomorrow while emphasizing the minor exception to the trend that always occur in Groups 13 and 16.
Test-taking tips for Regents and Honors Students:
For anything that you had to memorize for tomorrow's test, write out what you are afraid that you will forget repeatedly in order to put the information into your short-term memory. Then, AS SOON AS THE TEST BEGINS, write that information down on the test paper! If you know a good example of something that is related to what is on the test, WRITE THAT EXAMPLE down immediately on the test paper. This way, you can always remember and refer to your example at any time during the test. The one minute that you take at the beginning of the test to write these things down can save a lot of time and prevent errors on your test.
Also, label and annotate the key words in a given question; for example, if you see the word orbital in a question, DRAW ONE! Show that an orbital can hold a MAXIMUM of two opposite spin electrons.
Use these tips on any test that you take and you should do better.
Regents: We explained in terms of Zeff, OPEL's (just stick to those two reasons for all trends except for relating the size of an atom to its anion- that requires you to consider electron-electron repulsion within the valence shell of electrons) the Periodic Table trends in Ionization energy across a Period (form left to right) i.e. ionization energy increases across a period and ionization energy decreases down a group. We explained successive ionization energies and related that to the element's number of valence electrons. We discussed electronegativity and the Periodic trend in electronegativity across a Period and down a group.
The test tomorrow will cover all Periodic Table notes through today's discussion up to successive ionization energies (but not electronegativity). REVIEW and understand the notes, the homework and worksheets; then you will do well.
Honors: we discussed Mendeleev's formulation of the Periodic Table and the anomalies that he encountered because he did not know about electron configuration. At the end of class in D, I started to talk again about the different differences in energy between respective sublevels of different elements. I will draw that out better and more quantitatively on Friday.
Study (that means draw and write and then actually REFER TO YOUR DRAWING in your answers!) hard as you go over your notes and hw.
We then explained atom to ion size differences for cations and anions. We explained size differences among isoelectronic particles.
We began to explain the trend in ionization energy across a Period; we will continue to do so tomorrow while emphasizing the minor exception to the trend that always occur in Groups 13 and 16.
Test-taking tips for Regents and Honors Students:
For anything that you had to memorize for tomorrow's test, write out what you are afraid that you will forget repeatedly in order to put the information into your short-term memory. Then, AS SOON AS THE TEST BEGINS, write that information down on the test paper! If you know a good example of something that is related to what is on the test, WRITE THAT EXAMPLE down immediately on the test paper. This way, you can always remember and refer to your example at any time during the test. The one minute that you take at the beginning of the test to write these things down can save a lot of time and prevent errors on your test.
Also, label and annotate the key words in a given question; for example, if you see the word orbital in a question, DRAW ONE! Show that an orbital can hold a MAXIMUM of two opposite spin electrons.
Use these tips on any test that you take and you should do better.
Regents: We explained in terms of Zeff, OPEL's (just stick to those two reasons for all trends except for relating the size of an atom to its anion- that requires you to consider electron-electron repulsion within the valence shell of electrons) the Periodic Table trends in Ionization energy across a Period (form left to right) i.e. ionization energy increases across a period and ionization energy decreases down a group. We explained successive ionization energies and related that to the element's number of valence electrons. We discussed electronegativity and the Periodic trend in electronegativity across a Period and down a group.
The test tomorrow will cover all Periodic Table notes through today's discussion up to successive ionization energies (but not electronegativity). REVIEW and understand the notes, the homework and worksheets; then you will do well.
Honors: we discussed Mendeleev's formulation of the Periodic Table and the anomalies that he encountered because he did not know about electron configuration. At the end of class in D, I started to talk again about the different differences in energy between respective sublevels of different elements. I will draw that out better and more quantitatively on Friday.
Study (that means draw and write and then actually REFER TO YOUR DRAWING in your answers!) hard as you go over your notes and hw.
Tuesday, November 14, 2006
Tuesday
Honors: Today, we covered the simple formulas for determining , for a principal energy level, "n", the number of sublevels within that PEL = n, the number of orbitals within that PEL = n^2, and the maximum number of electrons of that PEL = n^2 * 2.
We defined "degenerate" orbitals. We also discussed "isoelectronic" particles such as Ne, F- , and Mg 2+ .
We then reviewed the physical significance of the terms: Principal Energy Level, Sublevel, and Orbital.
We viewed the electron density vs. distance from the nucleus video as well as the orbital/energy diagram video.
We then launched into the intro to the Periodic Table unit discussing the Law of Triads and the "Law of Eights or Octaves" as developed by Newlands and Mendeleev.
The Periodic Table info will not be on Thursday's exam BUT the above info about electron configurations, orbitals, isoelectronic particles, etc. will be.
E: we explained the periodic trends in atomic volume/size/radius and ionization energy. We also explained why all cations are smaller than their respective atoms and why all anions are larger than their respective atoms.
AP: we finished electron configurations and orbital diagrams of transition elements (valence electrons" first in, first out). Then we delicately discussed electron shielding and penetration within a given principal energy level: s electrons shield p,d,and f electrons, p electrons shield d and f electrons, and d electrons shield f electrons from some of the nuclear charge. "s" electrons are the most penetrating followed by p,d, and f electrons of a given principal energy level.
We began to explain the first of five or six major periodic trends. Of course, we will explain any and all exceptions in a perfectly normal, perfectly logical manner. Good times.
We defined "degenerate" orbitals. We also discussed "isoelectronic" particles such as Ne, F- , and Mg 2+ .
We then reviewed the physical significance of the terms: Principal Energy Level, Sublevel, and Orbital.
We viewed the electron density vs. distance from the nucleus video as well as the orbital/energy diagram video.
We then launched into the intro to the Periodic Table unit discussing the Law of Triads and the "Law of Eights or Octaves" as developed by Newlands and Mendeleev.
The Periodic Table info will not be on Thursday's exam BUT the above info about electron configurations, orbitals, isoelectronic particles, etc. will be.
E: we explained the periodic trends in atomic volume/size/radius and ionization energy. We also explained why all cations are smaller than their respective atoms and why all anions are larger than their respective atoms.
AP: we finished electron configurations and orbital diagrams of transition elements (valence electrons" first in, first out). Then we delicately discussed electron shielding and penetration within a given principal energy level: s electrons shield p,d,and f electrons, p electrons shield d and f electrons, and d electrons shield f electrons from some of the nuclear charge. "s" electrons are the most penetrating followed by p,d, and f electrons of a given principal energy level.
We began to explain the first of five or six major periodic trends. Of course, we will explain any and all exceptions in a perfectly normal, perfectly logical manner. Good times.
Monday, November 13, 2006
Monday
AP: we had our thermo/quantum exam and tomorrow we dig into the chemical periodicity unit. I will work on grading those exams tonight.
D/G Honors: we will have a written-response exam this Thursday covering everything about the Quantum Mechanical Model of the atom. Our last written test covered everything through the Bohr Model of the Hydrogen atom. Since then, we have discussed the evidence that led scientists to refine that model of the atom to our current Quantum/Wave-Mechanical Model of the atom. We covered principal energy levels, energy sublevels, and orbitals. We drew electron configurations, which showed the number of valence electrons per atom; we discussed dia- and paramagnetism as related to orbital diagrams. We developed orbital diagrams for the first four periods of elements by following the Aufbau Principle, the Pauli Exclusion Priniciple and Hund's Rule. We also thoroughly explained the exceptions to the Aufbau Principle that occur in Cr,Cu, Mo, and Ag in terms of electron distribution and relative energies of the 4s and 3d sublevels (or the 5s and 4d sublevels).
Today, we discussed electron transitions between energy sublevels; for example an electron transition from a 2s orbital to a 3d orbital as a result of an absorption of a specific quantum of energy. We COMPARED energy sublevels between atoms of different elements and noted NOT ONLY the different levels of energy that each respective sublevel has BUT ALSO the different differences in energy between respective sublevels of two different element's atoms; these two factors CAUSE the different emission (or absorption) spectra among the various elements.
In D, we started the Periodic Table unit noting the s, p, d, and f-block elements. We will get to that in G tomorrow.
E: we described and started to explain the Periodic Table trend of atomic radius/size/volume across a Period from left to right (atomic size DECREASES) and down a Group (atomic size increases). ALL trends will be explained in terms of THE MOST IMPORTANT THING IN ALL OF CHEMISTRY: Zeff, which is EFFECTIVE NUCLEAR CHARGE on an electron or electrons AND also the number of OCCUPIED (by electrons) PRINCIPAL ENERGY LEVELS. We will continue with these explanations tomorrow until we finish all six trends. You will see that each trend has THE SAME explanation. We will have our next test on Thursday; do your hw and make sure that you can easily answer questions such as the ones in today's class practice "quiz".
D/G Honors: we will have a written-response exam this Thursday covering everything about the Quantum Mechanical Model of the atom. Our last written test covered everything through the Bohr Model of the Hydrogen atom. Since then, we have discussed the evidence that led scientists to refine that model of the atom to our current Quantum/Wave-Mechanical Model of the atom. We covered principal energy levels, energy sublevels, and orbitals. We drew electron configurations, which showed the number of valence electrons per atom; we discussed dia- and paramagnetism as related to orbital diagrams. We developed orbital diagrams for the first four periods of elements by following the Aufbau Principle, the Pauli Exclusion Priniciple and Hund's Rule. We also thoroughly explained the exceptions to the Aufbau Principle that occur in Cr,Cu, Mo, and Ag in terms of electron distribution and relative energies of the 4s and 3d sublevels (or the 5s and 4d sublevels).
Today, we discussed electron transitions between energy sublevels; for example an electron transition from a 2s orbital to a 3d orbital as a result of an absorption of a specific quantum of energy. We COMPARED energy sublevels between atoms of different elements and noted NOT ONLY the different levels of energy that each respective sublevel has BUT ALSO the different differences in energy between respective sublevels of two different element's atoms; these two factors CAUSE the different emission (or absorption) spectra among the various elements.
In D, we started the Periodic Table unit noting the s, p, d, and f-block elements. We will get to that in G tomorrow.
E: we described and started to explain the Periodic Table trend of atomic radius/size/volume across a Period from left to right (atomic size DECREASES) and down a Group (atomic size increases). ALL trends will be explained in terms of THE MOST IMPORTANT THING IN ALL OF CHEMISTRY: Zeff, which is EFFECTIVE NUCLEAR CHARGE on an electron or electrons AND also the number of OCCUPIED (by electrons) PRINCIPAL ENERGY LEVELS. We will continue with these explanations tomorrow until we finish all six trends. You will see that each trend has THE SAME explanation. We will have our next test on Thursday; do your hw and make sure that you can easily answer questions such as the ones in today's class practice "quiz".
Friday, November 10, 2006
AP Exam Day 6

Our next exam, the first exam of the second quarter, will cover unit objectives that were posted on 10/29/06 EXCEPT for the last 5 objectives, the Periodicity objectives, which we haven't covered yet. Naturally, the class notes/examples should be the initial source for review. For explanations, be prepared to give detailed LABELED DRAWINGS/DIAGRAMS with accompanying explanations and examples, of
1. The Photoelectric effect and its use of the Planck equation
2. The Bohr Model of the Atom and its relation to the absorption and emission of quanta of energy.
3. The Quantum/Wave Mechanical Model of the Atom and its relation to the absorption/emission of quanta/photons; the relation of matter waves and the Heisenberg Uncertainty Principle to the development of the QM Model, specifically to the concept of "orbitals".
4. The exceptions to the Aufbau Principle i.e. Cr, Cu, Mo,and Ag.
For non-quantum review: expect a thermo problem that involves stoichiometry, calorimetry, and knowing the difference between enthalpy of formation vs. enthalpy of reaction. I've decided against another gas unit problem; sorry, we can only cover so much per test.
Another two test tips:
as we did in class, before you crunch numbers into a formula, write out EXPLICITLY (not just the symbols of the formula) what the formula is and how the variables relate to each other; for example, in the photoelectric effect problem, we wrote, "kinetic energy of the ejected electron = energy of the ABSORBED photon - energy required for the electron to overcome its attraction to the proton(s) in the nucleus"
This way, you'll place the measurements properly and you'll walk yourself through the problem.
ALSO, EXPLICITLY label your units. THE most frequent mess-up on the last test (and most tests) is to have a unit such as kJ per mol WITHOUT labeling what the mol IS!! There may be a HUGE difference between kJ per mol of REACTION and kJ per mol of whatever compound is in the reaction.
Also, it will be advantageous in most problems that have unit changes to work in Joules and kg because a Joule is a kg m^2 s^-2. To go from kJ to J, cross out the letter "k" in kJ and replace the letter with x 10^3 because that is literally what kilo means: 10^3.
On the past exams, students totally messed up and confused MATTER waves (for which you can ONLY use the deBroglie equation: wavelength of the "matter" = Planck's constant divided by the momentum of the matter-particle.) with electromagnetic radiation. ONLY PHOTONS can EVER travel at "c", the speed of light. All matter waves travel at less than the speed of light. Former classes were also unable to correctly do a "work function" photoelectric effect problem- I don't know. YOU all did one or two of them in class without a problem (it seemed). Some past students had trouble following the golden advice that they should draw and extensively label the drawing of the phenomena that they are about to explain. THEN, they should REFER to specific things in their drawing throughout the explanation. Instead, they thought that writing random facts qualified as an explanation. Untrue. Double untrue.
Thursday, November 09, 2006
Thursday
AP: today we covered the rules and EXCEPTIONS to the Aufbau Principle. We went through the electron configuration of the 4th Period elements and detailed why Cr and Cu do not exactly follow the Aufbau Principle. We also covered diamagnetic and paramagnetic atoms as related to orbital diagrams and electron configurations. All odd-atomic number atoms are paramagnetic because they MUST have at least one unpaired electron; SOME even-atomic number atoms are paramagnetic depending on whether or not they have at least one unpaired electron.
These orbital diagrams will be used to thoroughly explain the "periodicity" of the Periodic Table.
D/G:today we covered the EXCEPTIONS to the Aufbau Principle. We went through the electron configuration of the 4th Period elements and detailed why Cr and Cu do not exactly follow the Aufbau Principle. We discussed electron-electron repulsion and its effect on the potential energy of the electrons in the atom. We discussed "symmetric distribution of negatively charged electrons" (which results when a given SUBLEVEL is half-filled or fully filled) and how the symmetric arrangement causes the electrons to be, on average, FARther apart; this results in less OVERALL electron-electron repulsion which lowers their OVERALL potential energy.
E: we reviewed some of the properties of the elements and listened to the circus that was going on in the courtyard. We began to explain the SIX super-important trends across the periods and down the groups/families of the periodic table. These trends are outlined in the notes BUT make sure that you take very good notes on these explanations next week. We should cover enough ground to have a test or at least a quiz (shorter time for a quiz) on next Thursday.
These orbital diagrams will be used to thoroughly explain the "periodicity" of the Periodic Table.
D/G:today we covered the EXCEPTIONS to the Aufbau Principle. We went through the electron configuration of the 4th Period elements and detailed why Cr and Cu do not exactly follow the Aufbau Principle. We discussed electron-electron repulsion and its effect on the potential energy of the electrons in the atom. We discussed "symmetric distribution of negatively charged electrons" (which results when a given SUBLEVEL is half-filled or fully filled) and how the symmetric arrangement causes the electrons to be, on average, FARther apart; this results in less OVERALL electron-electron repulsion which lowers their OVERALL potential energy.
E: we reviewed some of the properties of the elements and listened to the circus that was going on in the courtyard. We began to explain the SIX super-important trends across the periods and down the groups/families of the periodic table. These trends are outlined in the notes BUT make sure that you take very good notes on these explanations next week. We should cover enough ground to have a test or at least a quiz (shorter time for a quiz) on next Thursday.
Wednesday, November 08, 2006
Mid-week
We began our second quarter today. This quarter has LOTS of vacation days (during which you'll be doing LOTS of chem, oh yes!), even more than the first quarter; yes, that is possible even though this is our fourth abbreviated week in a row. Don't fall behind because we have to cover a lot of ground in the least amount of time.
G/D Honors: today we wrote out the electron configurations and orbital diagrams for the first three periods of elements. Tomorrow, we get to the transition metals and we will see two quirky but explainable (yes, here comes an explanation question!) exceptions to the Aufbau Principle. I'll also show you the easy mnemonic so that you can correctly organize an atom in increasing sublevel energy order even if you don't have a periodic table nearby...1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s... you'll see the pattern tomorrow....
After that, we have a few easily deduced numerical relationships to see and then we can move on to the next unit: The Periodic Table= good times.
E: We're deep into the Periodic Table; today we learned various group names and various miscellaneous facts that appear on the Regents: allotropes of oxygen (O2 and O3 i.e. oxygen and ozone), allotropes of carbon = diamond, graphite, and buckyballs,
the room temp liquid METAL = Mercury = Hg and the room temp liquid non-metal = bromine = Br. We then discussed properties of metals vs. properties of non-metals: you guessed it, metals and non-metals have opposite characteristics.
AP: We continued with allowed quantum numbers that give the allowed quantized energies of an electron in an atom. We then did a photoelectric effect problem that last year's class couldn't do. Next, we started electron configurations in accord with the Aufbau Principle, the Pauli Exclusion Principle, and Hund's Rule.
We have a test when we come back from our three day weekend. I'll be at extra help tomorrow afternoon (as always) if you have practice questions that you want to go over. The test will be mostly on Quantum with a question or two about Thermo.
G/D Honors: today we wrote out the electron configurations and orbital diagrams for the first three periods of elements. Tomorrow, we get to the transition metals and we will see two quirky but explainable (yes, here comes an explanation question!) exceptions to the Aufbau Principle. I'll also show you the easy mnemonic so that you can correctly organize an atom in increasing sublevel energy order even if you don't have a periodic table nearby...1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s... you'll see the pattern tomorrow....
After that, we have a few easily deduced numerical relationships to see and then we can move on to the next unit: The Periodic Table= good times.
E: We're deep into the Periodic Table; today we learned various group names and various miscellaneous facts that appear on the Regents: allotropes of oxygen (O2 and O3 i.e. oxygen and ozone), allotropes of carbon = diamond, graphite, and buckyballs,
the room temp liquid METAL = Mercury = Hg and the room temp liquid non-metal = bromine = Br. We then discussed properties of metals vs. properties of non-metals: you guessed it, metals and non-metals have opposite characteristics.
AP: We continued with allowed quantum numbers that give the allowed quantized energies of an electron in an atom. We then did a photoelectric effect problem that last year's class couldn't do. Next, we started electron configurations in accord with the Aufbau Principle, the Pauli Exclusion Principle, and Hund's Rule.
We have a test when we come back from our three day weekend. I'll be at extra help tomorrow afternoon (as always) if you have practice questions that you want to go over. The test will be mostly on Quantum with a question or two about Thermo.
Tuesday, November 07, 2006
End of Quarter
Taking a break from grading right now; I hope to be done by midnight.
As the first quarter ends and we begin our new quarter with a new slate, you at least have the advantage of knowing how the class is run and what is expected of you.
The majority of students manage to improve and become better students, in general, as they continue to work hard and improve their reasoning and writing skills in subsequent quarters.
This does not have to be the case. In fact, among those who are doing poorly, I have noticed that very few of you (less than 5) have shown any desire to correct your errors. Students do not magically or suddenly become good at chemistry. They mainly become better at the subject by correcting past mistakes or preventing them in the first place (see previous "how to study" advice). So, for whatever you didn't "get" this past quarter, you should come in with some prepared questions and I'll walk you through some (illustrated) written explanations. You'll get much more out of extra help if you are aware of what you don't understand and, thus, come prepared with questions.
You'll see first quarter material throughout the year so it is important to make up for what you didn't get (or can't express) in order to succeed in upcoming quarters.
The last Honors written test was truly appalling. The results are even more unbelievable given the number of complete answers that I put online before the test (verbatim and extensive explanations of what we covered in class). The spelling and grammar from most students were atrocious; you will ALWAYS lose points for poor spelling and grammar. (Some are misspelling words that are IN THE QUESTIONS!) The questions forced you to draw pictures and diagrams which were then promptly ignored in your explanations. Information that was required for one question was given for a different question. Connections (such as "the energy of an emitted photon is EXACTLY equal to the energy lost by the electron; that SAME amount of energy equals Planck's constant times the frequency of light emitted") were ignored in lieu of just repeating information from the question. The bottom line is that too many students are not improving their writing, test-taking, and explanation skills. I suggest you come to extra help for that, OFTEN. Once you acquire the skill, it won't go away.
update: on the bright side, the lab exam, which is NO JOKE because you have to be super-careful with sig figs and units, looks very good. Quite a few 100s and most scored in the 90s.
As the first quarter ends and we begin our new quarter with a new slate, you at least have the advantage of knowing how the class is run and what is expected of you.
The majority of students manage to improve and become better students, in general, as they continue to work hard and improve their reasoning and writing skills in subsequent quarters.
This does not have to be the case. In fact, among those who are doing poorly, I have noticed that very few of you (less than 5) have shown any desire to correct your errors. Students do not magically or suddenly become good at chemistry. They mainly become better at the subject by correcting past mistakes or preventing them in the first place (see previous "how to study" advice). So, for whatever you didn't "get" this past quarter, you should come in with some prepared questions and I'll walk you through some (illustrated) written explanations. You'll get much more out of extra help if you are aware of what you don't understand and, thus, come prepared with questions.
You'll see first quarter material throughout the year so it is important to make up for what you didn't get (or can't express) in order to succeed in upcoming quarters.
The last Honors written test was truly appalling. The results are even more unbelievable given the number of complete answers that I put online before the test (verbatim and extensive explanations of what we covered in class). The spelling and grammar from most students were atrocious; you will ALWAYS lose points for poor spelling and grammar. (Some are misspelling words that are IN THE QUESTIONS!) The questions forced you to draw pictures and diagrams which were then promptly ignored in your explanations. Information that was required for one question was given for a different question. Connections (such as "the energy of an emitted photon is EXACTLY equal to the energy lost by the electron; that SAME amount of energy equals Planck's constant times the frequency of light emitted") were ignored in lieu of just repeating information from the question. The bottom line is that too many students are not improving their writing, test-taking, and explanation skills. I suggest you come to extra help for that, OFTEN. Once you acquire the skill, it won't go away.
update: on the bright side, the lab exam, which is NO JOKE because you have to be super-careful with sig figs and units, looks very good. Quite a few 100s and most scored in the 90s.
Monday, November 06, 2006
Monday Recap
AP and Honors, now it's your turn to...
ENTER THE ORBITRON!
and here is a great electron configuration tutorial
and a helpful video
Honors: we began the Aufbau Principle and encountered the (1) Pauli Exlusion Principle and (2) Hund's Rule.
On Wednesday, we will continue with more electron configuration, orbital diagrams, and practical questions and application of these representations of the QM Model of the Atom.
Regents: We further developed our picture of the Periodic Table. I'll show you some elements to support the descriptions of them.
AP: We got into the QM Model and are about to assign the FOUR quantum numbers per electron which details the exact energy and the probable location of each electron in an atom in the ground state.
ENTER THE ORBITRON!
and here is a great electron configuration tutorial
and a helpful video
Honors: we began the Aufbau Principle and encountered the (1) Pauli Exlusion Principle and (2) Hund's Rule.
On Wednesday, we will continue with more electron configuration, orbital diagrams, and practical questions and application of these representations of the QM Model of the Atom.
Regents: We further developed our picture of the Periodic Table. I'll show you some elements to support the descriptions of them.
AP: We got into the QM Model and are about to assign the FOUR quantum numbers per electron which details the exact energy and the probable location of each electron in an atom in the ground state.
Honors and Regents Lab Exam
Last year, the classes were VERY careful in their calculation on the lab test. Most of the students the knew significant figure rules inside and out, forwards and backwards. Be careful when you select your answer choice by MAKING ABSOLUTELY sure that the choice has the correct UNITS and the correct number of SIG FIGS.
MOST of the answer choices will be ALMOST correct, that mean that they are WRONG. One answer choice will be correct in units and the number of sig figs.
Fair warning.
Bottom line, know the objectives that were posted for your lab exam.
MOST of the answer choices will be ALMOST correct, that mean that they are WRONG. One answer choice will be correct in units and the number of sig figs.
Fair warning.
Bottom line, know the objectives that were posted for your lab exam.
Periodic Table
The Regents Class has recently begun the Periodic Table unit. Soon the Honors and AP classes will begin their respective Periodic Table units.
Regarding that, this website on the periodic table is an amazing compilation and (clearly) a labor of love.
http://www.theodoregray.com/PeriodicTable/index.html
Spend some time watching the vids or reading the stories and you'll have plenty of info to make life more interesting.
Regarding that, this website on the periodic table is an amazing compilation and (clearly) a labor of love.
http://www.theodoregray.com/PeriodicTable/index.html
Spend some time watching the vids or reading the stories and you'll have plenty of info to make life more interesting.
Saturday, November 04, 2006
server (temporary) trouble
I'm having difficulty connecting to our website server so, as a backup, I am posting the Honors and Regents classes' Lab exam objectives on Edline.com .
AP: I'm also posting, on Edline, the answers and a correction (the mass of the unknown acid was incorrectly listed as 1.000g when it should have been .1000 g) to the last review questions set (from 11/03/06).
As I type this, the server seems to be up again. Nevertheless, the backup files are on Edline just in case the site goes down again.
AP: I'm also posting, on Edline, the answers and a correction (the mass of the unknown acid was incorrectly listed as 1.000g when it should have been .1000 g) to the last review questions set (from 11/03/06).
As I type this, the server seems to be up again. Nevertheless, the backup files are on Edline just in case the site goes down again.
Weekend Diversion
Today is "Breeders' Cup" day: the biggest single day in horse racing (much bigger than Kentucky Derby day). On ESPN, you have the opportunity to view one of the greatest thoroughbred horses of the past 20 years. He is so good that he will be retired after today, win or lose (he has only lost once). Sadly, great horses are retired early because there is way more money to be made breeding top horses and selling their "babies" (foals that may or may not ever be good enough to compete) than there is money to be made by continuing racing and risking injury.
The superhorse who runs today is Bernardini and here he is:

ESPN @ 5PM. He will be challenged by some tough horses, Lava Man and Invasor, but, if Bernardini stays true to form, he will walk all over the field.
If you're not into horse racing, today's race will be the greatest introduction to the "Sport of Kings".
UPDATE: well, I guess that Bernardini is not a superhorse after all. Invasor easily beat him. Amazing that, just two races before he rode Bernardini, Bernardini's jockey was tossed to the ground at 45 mph; the jockey recovered and didn't seem affected after nearly being killed.
The superhorse who runs today is Bernardini and here he is:

ESPN @ 5PM. He will be challenged by some tough horses, Lava Man and Invasor, but, if Bernardini stays true to form, he will walk all over the field.
If you're not into horse racing, today's race will be the greatest introduction to the "Sport of Kings".
UPDATE: well, I guess that Bernardini is not a superhorse after all. Invasor easily beat him. Amazing that, just two races before he rode Bernardini, Bernardini's jockey was tossed to the ground at 45 mph; the jockey recovered and didn't seem affected after nearly being killed.
Friday, November 03, 2006
Honors Multiple Choice Results and Regents Class Update
D Period had a class average of 90.
G Period had a class average of 91 (I thought someone got a 19 but that person didn't use a number TWO pencil on the scantron!)
E Period, due to the corrected scantron, the class average on Thursday's Atomic Concepts 3 test was an 83. Getting even closer to the 90 goal! Good times.
Congratulations on the improved performance and good for you all (almost) for following the test-taking advice on the multiple choice exam. I know that, with proper practice, all classes can average even higher on the multiple choice lab exam on TUESDAY.
I will post the lab exam objectives when I get home (I hope) sometime tonight.
Have a good weekend!
G Period had a class average of 91 (I thought someone got a 19 but that person didn't use a number TWO pencil on the scantron!)
E Period, due to the corrected scantron, the class average on Thursday's Atomic Concepts 3 test was an 83. Getting even closer to the 90 goal! Good times.
Congratulations on the improved performance and good for you all (almost) for following the test-taking advice on the multiple choice exam. I know that, with proper practice, all classes can average even higher on the multiple choice lab exam on TUESDAY.
I will post the lab exam objectives when I get home (I hope) sometime tonight.
Have a good weekend!
AP lately
Thursday: we discussed the blackbody radiation results and how that led to Planck's Quantum Theory of Light; that theory was tested and found to be consistent with the evidence from the Photoelectric Effect.
FYI: Einstein won the Nobel Prize for this experiment and his interpretation of the results BUT HE DIDN'T win a Nobel for his mind-blowing Special Theory of Relativity AND (lightning struck TWICE) his General Theory of Relativity; E=mc^2, ever heard of that?? How many people have ever heard of the photoelectric effect? That's right, just you and me!!! What was UP with that Nobel committee??? I think I know why they didn't give him another prize and I think that there might be unsavory reasons for such an oversight.
We then applied Planck's equation mathematically/quantitatively.
Friday: we discussed bright-line emission spectra (visible and higher energy EMISSION SPECTRA DO NOT OCCUR AT ROOM TEMPERATURE! A sample has to be abnormally excited to produce the gamut of emitted photons!)
We related this evidence to Bohr's proposed Model of the atom. We discussed the mechanism of how this model matches the emission spectrum of hydrogen (video will be posted on the website!).
FYI: Einstein won the Nobel Prize for this experiment and his interpretation of the results BUT HE DIDN'T win a Nobel for his mind-blowing Special Theory of Relativity AND (lightning struck TWICE) his General Theory of Relativity; E=mc^2, ever heard of that?? How many people have ever heard of the photoelectric effect? That's right, just you and me!!! What was UP with that Nobel committee??? I think I know why they didn't give him another prize and I think that there might be unsavory reasons for such an oversight.
We then applied Planck's equation mathematically/quantitatively.
Friday: we discussed bright-line emission spectra (visible and higher energy EMISSION SPECTRA DO NOT OCCUR AT ROOM TEMPERATURE! A sample has to be abnormally excited to produce the gamut of emitted photons!)
We related this evidence to Bohr's proposed Model of the atom. We discussed the mechanism of how this model matches the emission spectrum of hydrogen (video will be posted on the website!).
Thursday, November 02, 2006
Regents: Stock is up today
More than half of the class scored above 85 so things are picking up again. Not bad for an "unannounced" exam. Class average was 81. We still have to pick up at least 9 points to call ourselves successful. Let's see that success next Tuesday on your last exam for the quarter: the Lab exam, which is a multiple choice test. Some of you improved significantly on today's test and I KNOW that the task was not easy. Congratulations to you for your effort.
ALMOST ALL who did not do well have not been to or are rarely at extra help; when you come to extra help, have questions ready. You will have questions ready if you are doing the homework and you are getting questions wrong OR if you are copying your notes and you do not understand them. People who come to extra help without questions will not improve nearly as much as they could if they came KNOWING what they do not comprehend. The absolute number one way to improve in chemistry is to, every DAY, REPEATEDLY do the different problem types that are tested until you can do them quickly and accurately every time (this also works beautifully in math).
It's time to change your priorities. I give extra help EVERY day FOR YOU. Whatever your excuses are for not going, if you don't change them, you're not magically going to improve. I hope that you will make a better effort for the second quarter.
ALMOST ALL who did not do well have not been to or are rarely at extra help; when you come to extra help, have questions ready. You will have questions ready if you are doing the homework and you are getting questions wrong OR if you are copying your notes and you do not understand them. People who come to extra help without questions will not improve nearly as much as they could if they came KNOWING what they do not comprehend. The absolute number one way to improve in chemistry is to, every DAY, REPEATEDLY do the different problem types that are tested until you can do them quickly and accurately every time (this also works beautifully in math).
It's time to change your priorities. I give extra help EVERY day FOR YOU. Whatever your excuses are for not going, if you don't change them, you're not magically going to improve. I hope that you will make a better effort for the second quarter.
Honors Multiple Choice Atomic Concepts Exam

...will be given on Friday. Expect approximately 33 multiple choice questions covering material from the last three written tests. Use the same test-taking skills: underlining/highlighting/circling keywords, answer EXACTLY what the question asks for.
ADVICE: The best way to take a multiple choice test is to treat it as a written test. Don't look at the answer choices until you either formulate or estimate an answer. Write or DRAW things out. The less you do in your head, the less chance that you'll make a careless error. Then, look for an answer choice that matches the answer from your work. If you don't find a matching answer, check that you read the question correctly.
You won't be graded on the work shown (other than identifying keywords) but you should show your work anyway to prevent yourself from making errors; don't do a problem only in your head, write it down instead.
Whenever you finish the test, you are required to work for the whole period, checking and re-checking your answers. If you got an answer one way, see whether you get the same answer another way. You may catch a careless error. You may catch a formal error.
Typically, this test is a nice grade booster (92 average last year) for those who study by doing lots of practice questions and who also show their work and do not misread the questions. Good luck tomorrow.
AP Quantum Links:
Here are two helpful sites that provide quantum atom unit animations, etc.
http://cwx.prenhall.com/bookbind/pubbooks/petrucci8/chapter9/deluxe.html
Click on "Student Tutorials" to see the list of animations.
This site is the bomb also:
http://highered.mcgraw-hill.com/sites/0073656011/student_view0/chapter7/elearning_session.html
http://cwx.prenhall.com/bookbind/pubbooks/petrucci8/chapter9/deluxe.html
Click on "Student Tutorials" to see the list of animations.
This site is the bomb also:
http://highered.mcgraw-hill.com/sites/0073656011/student_view0/chapter7/elearning_session.html
Wednesday, November 01, 2006
Regents Class
Your 4th Edition Barron's (Blue) Chem review book has some good review questions for Topic 7: The electronic structure of atoms. The questions are on pages 180-182; the relevant questions are numbers 1-35. Answers are found towards the back of the review book (see index).
I will collect the following homework (25 points towards your average) on Monday. It will be a useful review for your upcoming test also, so you may want to do this assignment tonight as a good test prep:
On looseleaf, for the first 36 elements on the periodic table, write the (1) name of the element, e.g. Aluminum, (2) the symbol of the element, e.g. Al, (3) the Bohr model electron configuration of the element ( e.g. 2-8-3) , (4) the quantum mechanical model electron configuration of the element (with s, p, d, etc...e.g. 1s2 2s2 2p6 3s2 3p1), and (5) the orbital diagram (labeled square boxes, with up and down arrows for opposite spin electrons) of the element. For electron configurations and for orbital diagrams, you may abbreviate the kernel/core electrons with the matching previous noble gas (group 18) in brackets, e.g. [Ne] 3s2 3p1.
Your paper(s) should have five columns (portrait or landscape format; landscape might be easier because it is wider than it is long), the first four columns should be much narrower than the last column because the orbital diagrams will take up the most space. Write NEATLY AND CLEARLY.
I will collect the following homework (25 points towards your average) on Monday. It will be a useful review for your upcoming test also, so you may want to do this assignment tonight as a good test prep:
On looseleaf, for the first 36 elements on the periodic table, write the (1) name of the element, e.g. Aluminum, (2) the symbol of the element, e.g. Al, (3) the Bohr model electron configuration of the element ( e.g. 2-8-3) , (4) the quantum mechanical model electron configuration of the element (with s, p, d, etc...e.g. 1s2 2s2 2p6 3s2 3p1), and (5) the orbital diagram (labeled square boxes, with up and down arrows for opposite spin electrons) of the element. For electron configurations and for orbital diagrams, you may abbreviate the kernel/core electrons with the matching previous noble gas (group 18) in brackets, e.g. [Ne] 3s2 3p1.
Your paper(s) should have five columns (portrait or landscape format; landscape might be easier because it is wider than it is long), the first four columns should be much narrower than the last column because the orbital diagrams will take up the most space. Write NEATLY AND CLEARLY.
Honors Prep
Thanks to those who are studying for the Honors exams on Thursday and Friday. To share the wealth, I corrected some of the submitted responses and posted them on the class website. I won't always have time to do so, but I have the day off so I have the opportunity to undo some damage BEFORE the test.
Don't forget: CAREFULLY READ each question so that you understand exactly what IS being asked for. Then, your absolute knee-jerk response by now should be NOT TO WRITE!!!, BUT RATHER, to draw and label a detailed LARGE (go LANDSCAPE, if you must) picture. THEN, write/elaborate on what you drew (often that elaboration of your drawing IS an explanation if it shows how and why things are occurring).
Watch the videos and see the pictures from the powerpoints!
Don't forget: CAREFULLY READ each question so that you understand exactly what IS being asked for. Then, your absolute knee-jerk response by now should be NOT TO WRITE!!!, BUT RATHER, to draw and label a detailed LARGE (go LANDSCAPE, if you must) picture. THEN, write/elaborate on what you drew (often that elaboration of your drawing IS an explanation if it shows how and why things are occurring).
Watch the videos and see the pictures from the powerpoints!