Wednesday, December 28, 2005
Atoms vs. Atoms and Isotopes

Honors: As some have noticed, the "Atoms" and "Atoms and Isotopes" Modules are quite redundant. You do not have to answer the same questions twice.
Cheers.
Tuesday, December 20, 2005
Vacation Assignments

Our short-awaited vacation is finally here! Good times. This year is flying by and we will return strong in '06. I hope to have all of your tests graded by then and I hope to see many perfect or high scores.
Naturally, we have some hw over the vacation: reinforcement material for Honors so that you can recall what you already know; some new and some review material for AP.
Honors: Download each "learning module" powerpoint (you must email me if you have any trouble doing so), read the lessons, write the questions and answer the questions BEFORE you look at the answer(s) as you go through the powerpoints. Show any necessary work (reasoning or calculation) that you did to get your answer. It's fine if you get the problem wrong. You can just put a line through your original answer and then write in the correction. Don't cheat yourself out of doing this properly; you will see some of these questions on upcoming exams. Label and organize your papers before you hand them in.
AP: It's all you for Chapters 11 and 12. Don't hesitate to email me if you have a question on how to do something but make sure that you first have read the text/study guides and looked at/solved the sample questions.
Later, I will post some more solved problems which will be categorized by question type and I will post a link or two to help guide you.
There will be two tests soon after the vacation: one on Chapters 11 and 12 (be ready for that within the first few days back to school - 40 minutes for that test!) and then one on Chapter 10 (which will be on our next day 4).
Wednesday, December 14, 2005
Upcoming exams
The next Honors Chem exam will be on Monday and Tuesday. Tuesday's class time is only 20 minutes so the question or questions that day will not be long. Unlike the last exam, you will not get to see Monday's questions again on Tuesday. That is, you must finish Monday's questions in class on Monday.
The test will cover material from our Bonding unit. We will probably finish naming compounds and possibly drawing Lewis structures (the quicker and easier electron-"counting" method) by Friday.
AP: Monday is day 4 which means our Bonding I test (yes! not as much writing as the last "thesis-length" test!). We should be done with Lewis structures and some/most of intra and inter-particle attractions by then so, on Friday, I will let you know what to expect for Monday's exam.
There is a TWO unit Christmas vacation assignment that you will be tested on soon after the break. I will be posting many questions with solutions to help you through those units but you will have to practice the problems to understand how to apply the material in those two chapters.
The test will cover material from our Bonding unit. We will probably finish naming compounds and possibly drawing Lewis structures (the quicker and easier electron-"counting" method) by Friday.
AP: Monday is day 4 which means our Bonding I test (yes! not as much writing as the last "thesis-length" test!). We should be done with Lewis structures and some/most of intra and inter-particle attractions by then so, on Friday, I will let you know what to expect for Monday's exam.
There is a TWO unit Christmas vacation assignment that you will be tested on soon after the break. I will be posting many questions with solutions to help you through those units but you will have to practice the problems to understand how to apply the material in those two chapters.
Naming Compounds: Practice^3
Check out the compound-naming practice link in the upper left corner of this blog.
http://dbhs.wvusd.k12.ca.us/webdocs/Nomenclature/Nomenclature.html
Here is a site that gives a nice, simple overview of bonding along with some quizzes:
http://www.bbc.co.uk/schools/gcsebitesize/chemistry/classifyingmaterials/ionic_bondingrev1.shtml
http://dbhs.wvusd.k12.ca.us/webdocs/Nomenclature/Nomenclature.html
Here is a site that gives a nice, simple overview of bonding along with some quizzes:
http://www.bbc.co.uk/schools/gcsebitesize/chemistry/classifyingmaterials/ionic_bondingrev1.shtml
Friday, December 09, 2005
Weekend assignments
The Honors worksheets contain review material on ionic bonding. Just answer up to and including question 7 on the "Activity 4-5: The Chemical Bond II" worksheet.
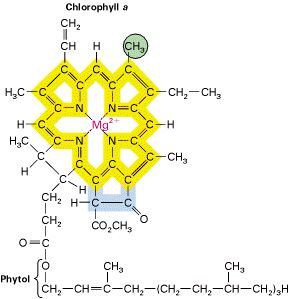
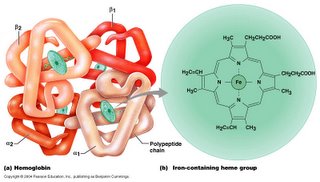
AP: Transition Metal- Ligand Complexes is one of the self-study units in our course (which will help you answer a couple of the "weed-out" multiple-choice questions on the AP exam). The difficult part of the unit is to facilely name these daunting compounds. You MUST practice naming them repeatedly until you can quickly do so. The chapter is one of the most interesting in chemistry (so read the whole chapter if you desire) and you will deal almost exclusively with these complex compounds if you take inorganic chemistry in college; some of the most biologically vital compounds are metal-ligand complexes: hemoglobin (left), chlorophyll (above) anyone?


AP: Transition Metal- Ligand Complexes is one of the self-study units in our course (which will help you answer a couple of the "weed-out" multiple-choice questions on the AP exam). The difficult part of the unit is to facilely name these daunting compounds. You MUST practice naming them repeatedly until you can quickly do so. The chapter is one of the most interesting in chemistry (so read the whole chapter if you desire) and you will deal almost exclusively with these complex compounds if you take inorganic chemistry in college; some of the most biologically vital compounds are metal-ligand complexes: hemoglobin (left), chlorophyll (above) anyone?
Today's Math Lesson
Friday + cold = No school.
:)
I foresee many three-day weekends this year, which is nice, but that means that we will be under tremendous pressure to finish our coursework and review on time. We only have six days of school until 2006.
Thus, check the website later today for the weekend AP and Honors assignments that I will collect on Monday (Honors) and Tuesday (AP).
:)
I foresee many three-day weekends this year, which is nice, but that means that we will be under tremendous pressure to finish our coursework and review on time. We only have six days of school until 2006.
Thus, check the website later today for the weekend AP and Honors assignments that I will collect on Monday (Honors) and Tuesday (AP).
Monday, December 05, 2005
Set your TiVo for some METAL
Next week, the Discovery Channel is hosting a bunch of shows on the myriad uses of various elements. Check out the preview page:
http://school.discovery.com/ontv/themes/f2005_periodicTable.html#tuesday
Now you finally have a reason not to watch MTV.
:)
http://school.discovery.com/ontv/themes/f2005_periodicTable.html#tuesday
Now you finally have a reason not to watch MTV.
:)
Snow Patrol...
Three possibilities for tomorrow with three different respective game plans:
1. No School: plan - sleep. have fun. do some hw: AP- Read Section 9.1 of the text and continue to study for the day 4 test which would be on Wednesday. Honors- answer text section 7.1 review questions and then read and outline section 7.2
2. Delayed School opening: AP -we will have the multiple choice section of our Periodicity test for our 55 minutes of class time. Honors- we will quickly continue our Bonding unit for 25 minute periods.
3. Regular school day...we sigh and then get back to work...and like it.
Honors Multiple Choice test averages:
D: 95
E: 95
B: 91
very good work all... (D was the winner by 0.4 over E...very close!).
1. No School: plan - sleep. have fun. do some hw: AP- Read Section 9.1 of the text and continue to study for the day 4 test which would be on Wednesday. Honors- answer text section 7.1 review questions and then read and outline section 7.2
2. Delayed School opening: AP -we will have the multiple choice section of our Periodicity test for our 55 minutes of class time. Honors- we will quickly continue our Bonding unit for 25 minute periods.
3. Regular school day...we sigh and then get back to work...and like it.
Honors Multiple Choice test averages:
D: 95
E: 95
B: 91
very good work all... (D was the winner by 0.4 over E...very close!).
Sunday, December 04, 2005
Nice AP practice link
This is a link (provided by the godfather of all AP chem teachers, Prof. Adrian Dingle, from that GA prep school) to some short and sweet (5 questions per quiz) multiple choice AP-level questions. The quizzes are graded immediately for instant feedback and gratification. I will make this link permanent on the side links also.
http://www.adriandingleschemistrypages.com/apquiz.html
http://www.adriandingleschemistrypages.com/apquiz.html
Friday, December 02, 2005
Honors Periodic Table MC exam early results
I accepted two answers to question 26 because it is unfair to be asked to compare the size of a fluoride ion to the size of a calcium (2+) ion. Those particles have different Zeffs on their outermost PEL electrons and ALSO a different number of OPEL's. If you must know, the experimental data show that the calcium 2+ ion is about 37 pm smaller but I certainly do NOT want you to memorize or guess that.
p.s. as a result, everyone got that question right and there were at least six more perfect scores.
There also an active metal question (Na vs. Ca) that is a close call so I am accepting both answers ( though sodium is a bit more active ). Good times.
That said, the results are (approximate until I enter them in my excel gradebook):
D and E Periods: 93 ( oh! it's on!)
B Period: 90
These averages will increase once I make the above corrections.
Good work! Have a great weekend!
p.s. as a result, everyone got that question right and there were at least six more perfect scores.
There also an active metal question (Na vs. Ca) that is a close call so I am accepting both answers ( though sodium is a bit more active ). Good times.
That said, the results are (approximate until I enter them in my excel gradebook):
D and E Periods: 93 ( oh! it's on!)
B Period: 90
These averages will increase once I make the above corrections.
Good work! Have a great weekend!